Peking The Projects
From 2007.igem.org
Qu Mingzhi (Talk | contribs) m (→Push-on-push-off Switch) |
(→Measuring the NOR Gates) |
||
| Line 61: | Line 61: | ||
[[Image:Peking Swich fig.6.JPG|500px]] | [[Image:Peking Swich fig.6.JPG|500px]] | ||
*Based on the results above, we choose SD1, SD2, SD3 to have further testing. A plasmid with LacI is transformed into CAJ64. IPTG is added prior to UV irradiation, to vary the effective level of LacI. As shown in Fig 7, SD2 behaves most similar to a NOR Gate. | *Based on the results above, we choose SD1, SD2, SD3 to have further testing. A plasmid with LacI is transformed into CAJ64. IPTG is added prior to UV irradiation, to vary the effective level of LacI. As shown in Fig 7, SD2 behaves most similar to a NOR Gate. | ||
| - | [[Image:Peking Swich | + | [[Image:Peking Swich fig7b.JPG|500px]] |
| + | |||
====Summary ==== | ====Summary ==== | ||
We have constructed and tested the '''Bistable Switch''' and '''NOR Gate''' which are the basic elements of the push-on push-off switch. The mathematical model also sheds light on how to connect these two elements together to make the circuit work. The relative abundance of various regulators can be fine-tuned by changing the SD signals which we have measured. Our next step would be combine the two parts and realize an integrate system working as expected | We have constructed and tested the '''Bistable Switch''' and '''NOR Gate''' which are the basic elements of the push-on push-off switch. The mathematical model also sheds light on how to connect these two elements together to make the circuit work. The relative abundance of various regulators can be fine-tuned by changing the SD signals which we have measured. Our next step would be combine the two parts and realize an integrate system working as expected | ||
Revision as of 01:09, 26 October 2007
| Peking | The Projects | The Team | Misc & Fun | [http://openwetware.org/wiki/IGEM:Peking/2007 Peking OWW] | Thanks |
|---|
Hop Count with Conjugation
Hop counting is a cell-cell communication system in which the signals transfered among the cells record the number of times of transfer. we have adapted natural conjugation systems as the communication device, and the conjugative plasmids as the vector for the transfered signal. With the rolling-cycle replication of conjugation, independent tandem oriT (BBa_J01002 and more) pairs can be inserted into the same plasmids, losing one tandem region at each single conjugation event. Sequential conjugation and deletion of the different oriT pairs can be achieved by fine control of the expression of the cis-acting elements of the independent conjugation systems.Besides intellectual interests, these systems are the core parts of controllable and encoded local cell-cell communication, which can be used in potential pattern formation and material weaving applications.
Push-on-push-off Switch
Abstract:
We are going to engineer the E. coli cell to behave as a push-on push-off switch. We have designed a gene regulatory circuit that senses Ultra-Violet light (UV) irradiation as input signal. Under intermittent irradiation, two reporter genes are to be induced alternately. The circuit can be divided into two associated parts: a bistable switch and a NOR gate. The NOR gate is controlled by the bistable switch and the input signal. On the other hand, the output of the NOR gate can switch the bistable state from one to the other. Thus far, we have constructed a series of NOR gates and one bistable switch.
Achievement:
Gene Circuit Components:
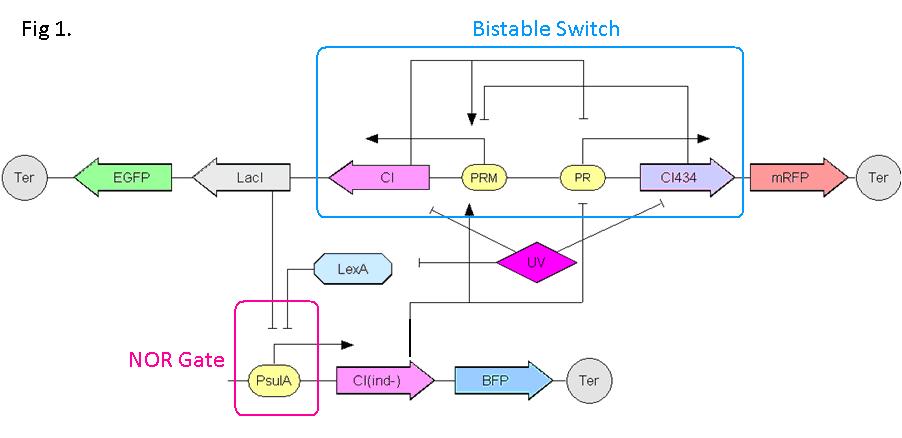
Fig.1 shows the scheme of the gene circuit. The Bistable Switch is composed of CI and CI434 which repress each other in transcription. The NOR Gate is a promoter repressed by both LacI and LexA. These two parts are connected by LacI and CI(ind-). UV irradiation induces SOS response in E. coli, causing rapid degradation of LexA, CI and CI434. CI(ind-) is a mutation of CI which functions the same as CI but is not readily degraded in SOS response.
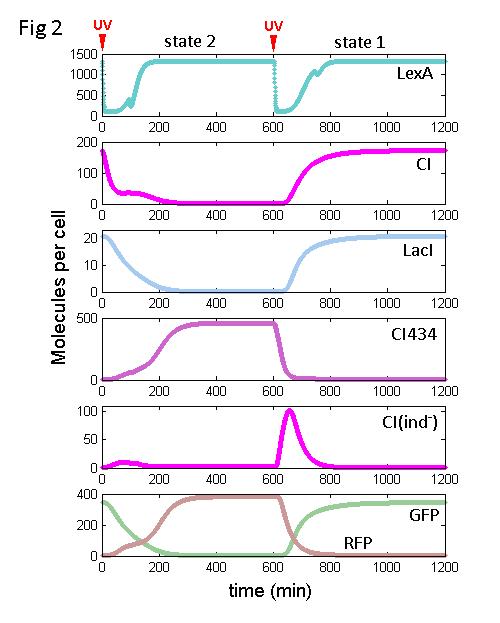
Mathematical model
A set of ordinary differential equations (ODEs) are used to describe the dynamic behavior of this circuit after UV irradiation. As shown in Fig 2, initially the cell is expressing CI-GFP-LacI. CI434-RFP is repressed by CI, and CI(ind-) is repressed by both LexA and LacI. We call this state 1. When the cell is exposed to UV irradiation, LexA and CI are degraded. In the absence of CI, LacI transcription attenuates. Meanwhile, LacI concentration decreases gradually owning to the cell growth. At the same time, as the DNA damage produced by UV being repaired, LexA, CI, and CI434 tend to re-accumulate. As a result, during the SOS response and thereafter, CI(ind-) is always repressed, firstly by LacI and then by LexA. At this moment, because the promoter PR is stronger than PRM, CI434 accumulates faster and represses CI expression. The cell turns to state 2, in which CI434-RFP is high and CI-GFP-LacI is low.
When the cell is exposed to UV irradiation again, LexA is degraded, and in the absence of both LexA and LacI, CI(ind-) is expressed. Before CI(ind-) is turned off by LacI and LexA, it helps to repress CI434, and induce the expression of CI, turning the cell back to state 1.
According to the simulation result, the circuit does have the potential to behave like a push-on push-off switch.
Constructing the Bistable Switch
We modify the wild type PRM/PR region of lambda phage so that it can be repressed by CI and CI434 in opposite transcription direction. As shown in Fig 3(a), two different promoter designs are considered. The first is to replace OR3 with two CI434 binding site, while the second is to add them after OR3. Three SD signals are selected as candidates. pSB3K3 plasmid is chosen to carry the switch. The plasmid is transformed into strain DH5alpha. The culture is then plated over night. It turns out that the first promoter design shows bistable characteristic, namely, a fraction of the colons express RFP while others express EGFP (Fig 3 (b) and (c)).
Constructing the NOR Gate
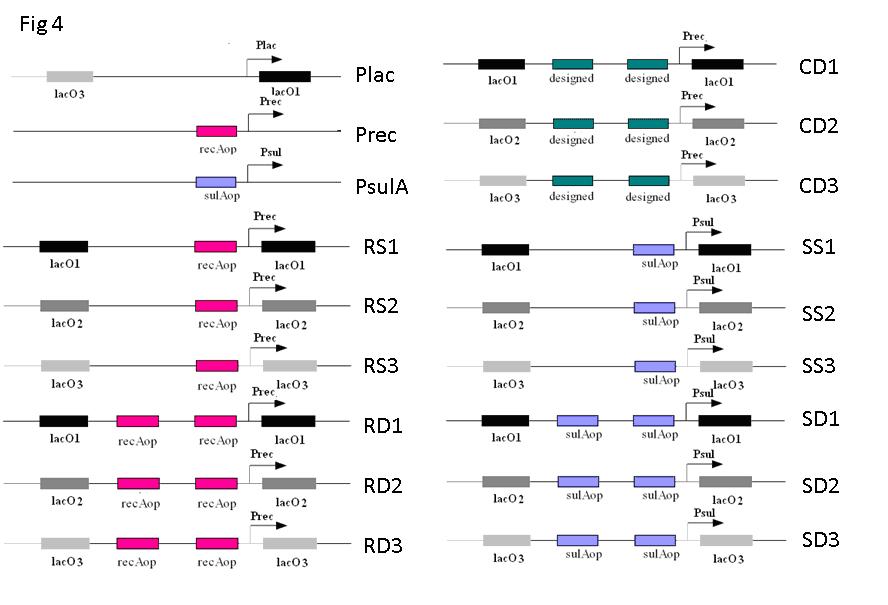
We combine the SOS gene promoter with LacI binding site so that it can be repressed by both LacI and LexA. In order to obtaining a satisfying NOR gate, we make 15 combinations to cover various possibilities. As shown in Fig 4, LacO1, LacO2, LacO3 are LacI binding sites of different affinity. recAop and sulAop are LexA binding sites from two SOS genes, sulA and recA respectively. ‘designed’ is the most consensus LexA binding site. Plac, Prec, PsulA are wild type promoters (from -35 region to +1) of lac operon, recA and sulA respectively. EGFP is constructed downstream to these promoters in plasmid pLX007, serving as a reporter.
Measuring the NOR Gates
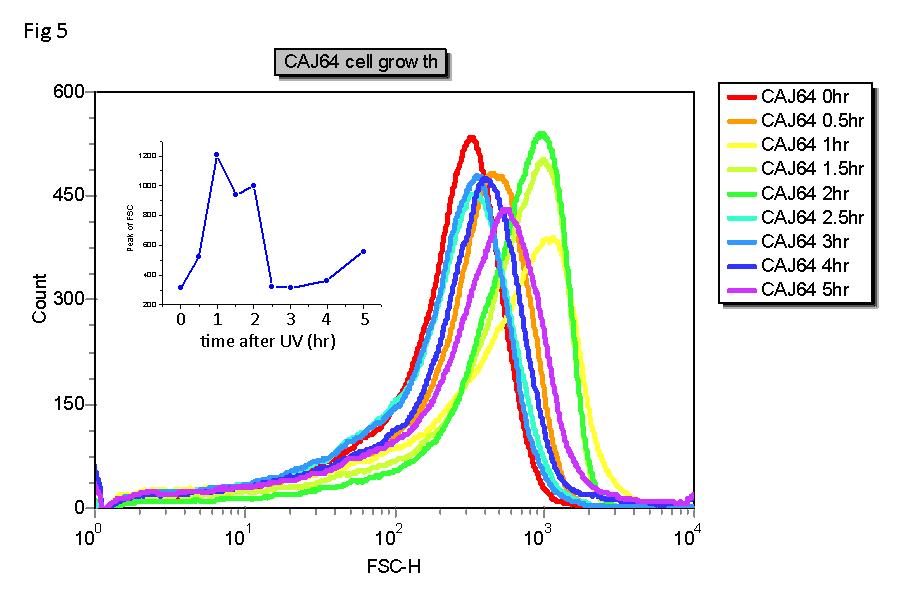
The plasmid is transformed into strain CAJ64 ((del)(colB-lacI)3, relA1, spoT1, trpT171(UGAs)). The culture is irradiated in mid-log phase. Thereafter, samples are taken every half hour. The cell length and EGFP level are measured by FACS.
- SOS response in CAJ64. During SOS response, the length of the cell correlates with LexA protein level. When LexA level is low, a SOS gene sulA is activated and represses cell division, so that the cell grows longer. From Fig 5, we can infer that LexA level decreases within half an hour and starts to re-accumulate after 2 hours.
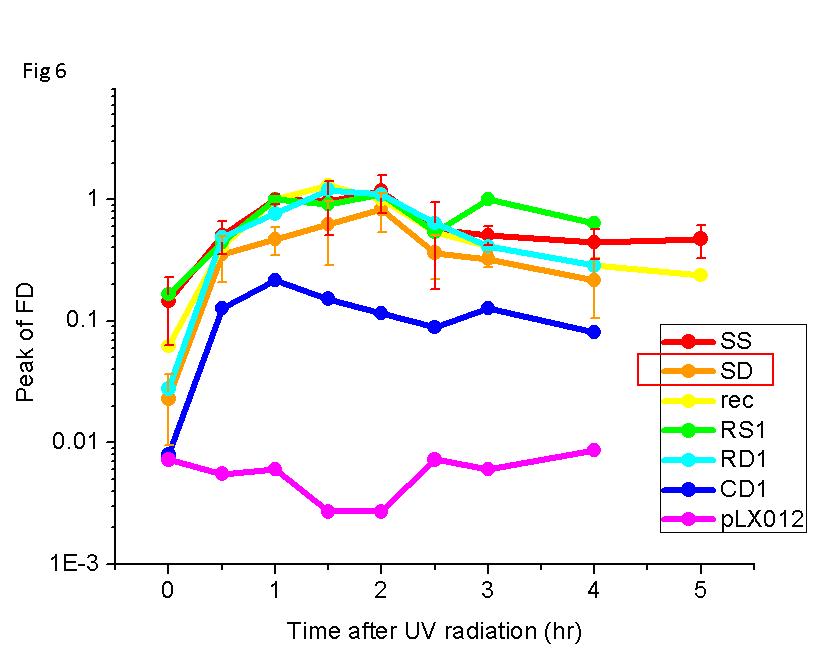
- In order to compare ‘sulAop’, ‘recAop’ and ‘designed’, we measure the response curve in the absence of LacI. As shown in Fig 6, the promoter with two ‘sulAop’ has both large induction fold and high expression level.
- Based on the results above, we choose SD1, SD2, SD3 to have further testing. A plasmid with LacI is transformed into CAJ64. IPTG is added prior to UV irradiation, to vary the effective level of LacI. As shown in Fig 7, SD2 behaves most similar to a NOR Gate.
Summary
We have constructed and tested the Bistable Switch and NOR Gate which are the basic elements of the push-on push-off switch. The mathematical model also sheds light on how to connect these two elements together to make the circuit work. The relative abundance of various regulators can be fine-tuned by changing the SD signals which we have measured. Our next step would be combine the two parts and realize an integrate system working as expected
Email us at igem-pku AT googlegroups DOT com
External links iGEM | iGEM 2007 | Team List | [http://partsregistry.org Parts] | [http://openwetware.org/wiki/IGEM:Peking/2007 Peking OWW] | [http://www.pku.edu.cn Peking University]