ETHZ/Biology/Lab
From 2007.igem.org
Introduction
On this page, you can find information about how educatETH E.coli was implemented in the lab. More specifically, you will find information on the plasmid strains we used, the modifications we did to them in order to be compatible with the Biobrick library and our cloning plan. Moreover, you can find here an (unfortunately not complete) electronic copy of our lab notebook. If you are here because you are interested in implementing educatETH E.coli in your lab, then our System Implementation and the System Parts pages may be of help to you!
For all our cloning procedures we used standard protocols according to SAMBROOK and RUSSELL Molecular Cloning: A Laboratory Manual [1].
Strains
We used the following E. coli strains:
- You can find this strain at BBa_V1009
- This strain has a streptomycin resistance
- Genotype: F’ {tetR}, mcrA, Δ(mrr-hsdRMS-mcrBC), φ80 lacZ ΔM15, ΔlacX74, deoR, recA1, araD139 Δ(ara-leu)7679, galU, galK, λ-, rpsL,endA1, nupG
- For further information please click here
- References:
- Casdaban, M. and Cohen, S. (1980) J Mol Biol 138:179 PMID 6997493
- Grant, S.G.N. et al. (1990) Proc. Natl. Acad. Sci. USA 87: 4645-4649 PMID 2162051
- Casdaban, M. and Cohen, S. (1980) J Mol Biol 138:179 PMID 6997493
- You can find this strain at BBa_I739301
- We call them Jimmys
- This strain is the original blue/white cloning strain
- Genotype: glnV44, thi-1, Δ(lac-proAB), F'[lacIqZΔM15 traD36 proAB+]
- For further information please click here
- Reference:
- Messing, J. et al. (1981) Nucleic Acids Res. 9, 309; Yanisch-Perron, C., Vieira, J., and Messing, J. (1985) Gene 33, 103
Plasmids
For our system we needed three plasmids with different origins of replication and antibiotic resistances. We decided to take low copy plasmids. We also decided to use the following plasmids, which we wanted to modify so that they would become compatible to the Biobrick Library multiple cloning site:
Basic plasmids
| Plasmid | Resistances | Copy number | Origin | Map |
|---|---|---|---|---|
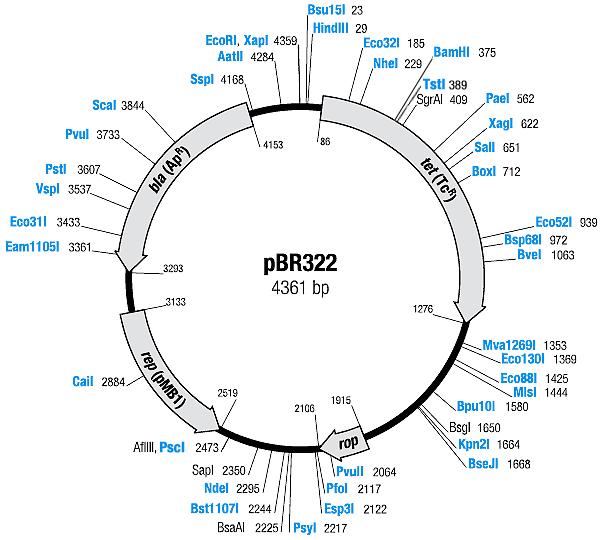
| pBR322 [2,3] | Ampicillin, Tetracyline | 15-20 [4] | pMB1 | |
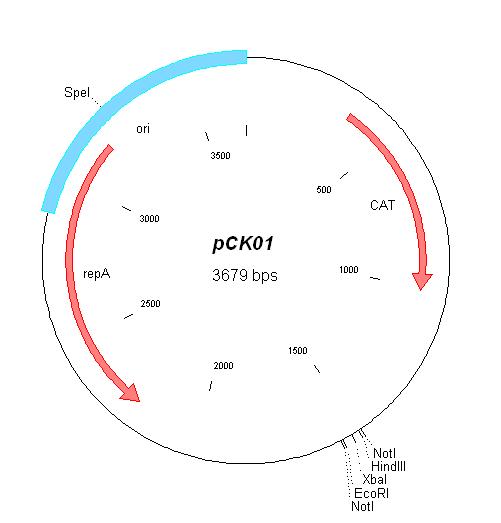
| pCK01 [5] | Chloramphenicol | 5-12 [4] | pSC101 | |
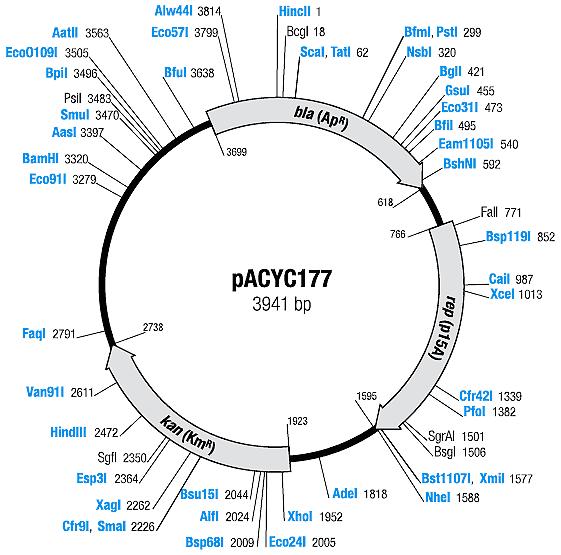
| pACYC177 [6,7] | Ampicillin, Kanamycin | 10-12 [4] | p15A |
Changes to the plasmids
In order to get the Biobrick multiple cloning site into the plasmids, we had to make several changes to the plasmids:
| Plasmid | Changes | New name | New resistance | New Map |
|---|---|---|---|---|
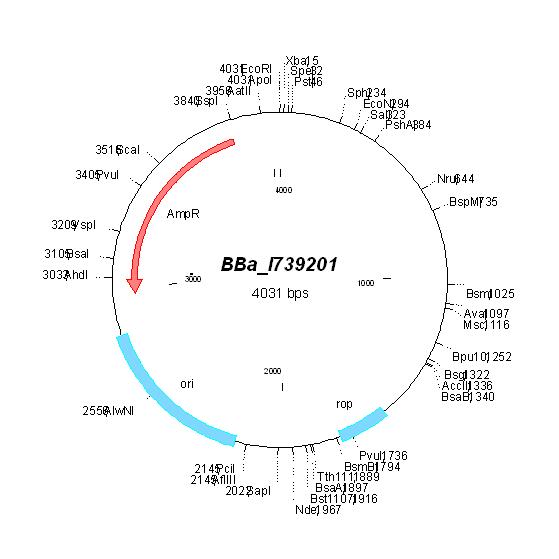
| BBa_I739201 |
|
Ampicillin | ||
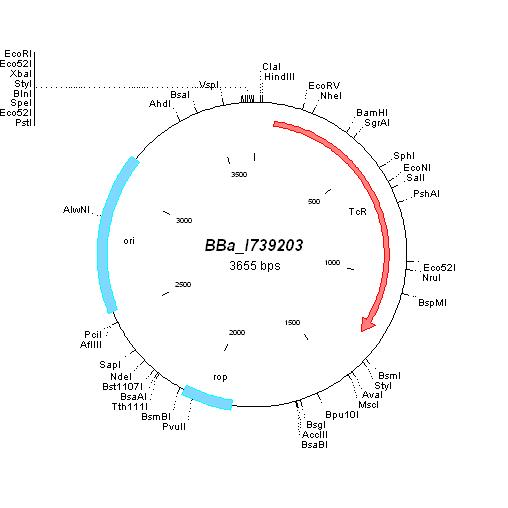
| BBa_I739203 |
|
Tetracycline | ||
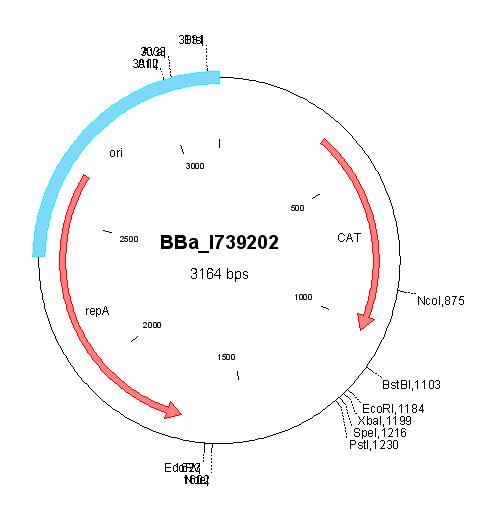
| BBa_I739202 |
|
Chloramphenicol | ||
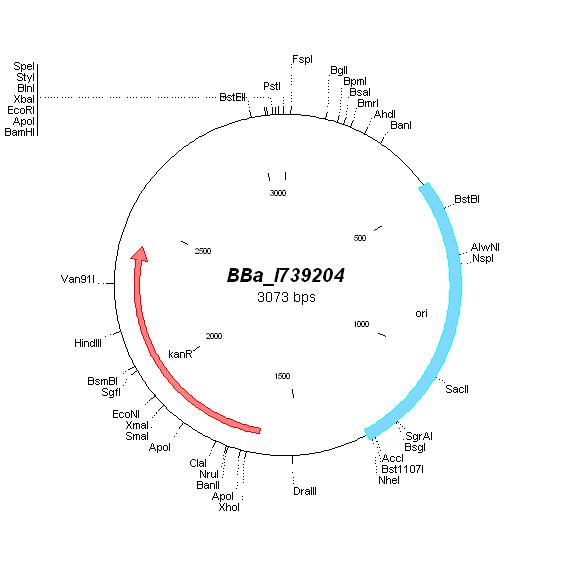
| BBa_I739204 |
|
Kanamycin |
Cloning plan
Parts assignment into plasmids
The plan was to put the following parts into the three plasmids (for the detailed cloning procedure see below):
| plasmid | resistance | copy type | contents | comments |
|---|---|---|---|---|
| BBa_I739201 | ampicillin | low | BBa_I739001(TetR) , BBa_I739002(LacI) , BBa_I739003(LuxR) | constitutive subsystem |
| BBa_I739202 | chloramphenicol | low | BBa_I739004(P22 cII) , BBa_E0430(EYFP) , BBa_I739008(cI) , BBa_I739009(ECFP) | reporting subsystem |
| BBa_I739204 | kanamycin | low | BBa_I739006(cI) , BBa_I739007(P22 cII) , BBa_I739010(RFP) , BBa_I739011(GFP) | learning subsystem, reporting subsystem |
It is important to insert parts responsible for the production of fluorescent proteins in low copy plasmids, as they are potentially harmful for the cell. Unfortunately, working with low copy plasmids makes the procedure more demanding in the lab.
Cloning procedure
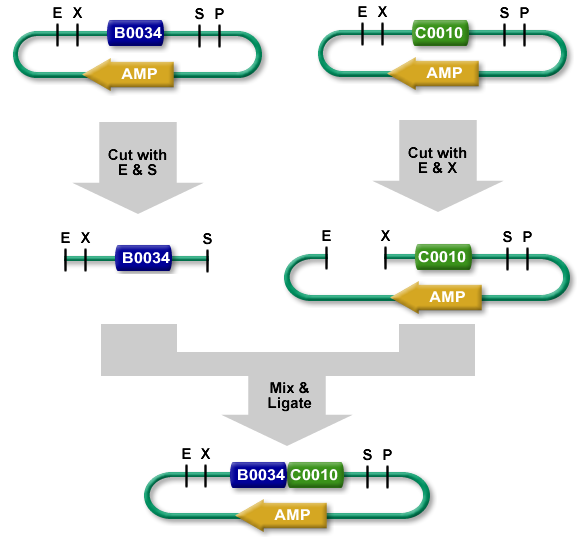
The standard BioBrick assembly will be used to put the parts in the plasmids. Detailed information on how the BioBrick part fabrication works can be found here. For a shorter explanation of how to assemble 2 parts together check here. Note that the composite part is constructed from the end to the beginning, i.e. each new part is inserted before the existing one. Composite parts made of parts a and b are denoted a.b.
Plasmid 1 (BBa_I739201)
- Digest I739001(TetR) and BBa_I739201 plasmid with EcoRI/PstI and ligate them afterwards.
- Digest I739002(LacI) and I739003(LuxR) with XbaI/PstI
- Digest I739001(TetR) in BBa_I739201 with SpeI/PstI.
- Ligate digested I739001(TetR) in BBa_I739201 with digested I739002(LacI). You get a plasmid containing a I739001(TetR).I739002(LacI) composite part.
- Digest I739001(TetR).I739002(LacI) with SpeI/PstI.
- Ligate digested I739001(TetR).I739002(LacI) with digested I739003(LuxR). You get the completed I739001(TetR).I739002(LacI).I739003(LuxR) composite part in the BBa_I739201 plasmid.
Plasmid 2 (BBa_I739202)
- Digest BBa_I739004(P22 cII), BBa_I739008(cI) and the plasmid BBa_I739202 with EcoRI/PstI.
- Digest BBa_E0430(EYFP) and BBa_I739009(ECFP) with XbaI/PstI.
- Ligate digested BBa_I739004(P22 cII) and digested plasmid BBa_I739202.
- Ligate digested BBa_I739008(cI) and the plasmid BBa_I739202.
- Digest BBa_I739004(P22 cII) in BBa_I739202 with SpeI/PstI.
- Digest BBa_I739008(cI) in BBa_I739202 with SpeI/PstI.
- Ligate digested BBa_I739004(P22 cII) in BBa_I739202 with digested BBa_E0430(EYFP). You get a plasmid containing a BBa_I739004(P22 cII).BBa_E0430(EYFP) composite part.
- Ligate digested BBa_I739008(cI) in BBa_I739202 with BBa_I739009(ECFP). You get a plasmid containing a BBa_I739008(cI).BBa_I739009(ECFP) composite part.
- Digest BBa_I739004(P22 cII).BBa_E0430(EYFP) with SpeI/PstI.
- Digest BBa_I739008(cI).BBa_I739009(ECFP) with XbaI/PstI.
- Ligate digested BBa_I739004(P22 cII).BBa_E0430(EYFP) and digested BBa_I739008(cI).BBa_I739009(ECFP). You get the completed plasmid containing the BBa_I739004(P22 cII).BBa_E0430(EYFP).BBa_I739008(cI).BBa_I739009(ECFP) composite part.
Plasmid 3 (BBa_I739204)
- Digest BBa_I739006(cI), BBa_I739010(RFP) and the plasmid BBa_I739204 with EcoRI/PstI.
- Digest BBa_I739007(P22 cII) and BBa_I739011(GFP) with XbaI/PstI.
- Ligate digested BBa_I739006(cI) and digested plasmid BBa_I739204.
- Ligate digested BBa_I739010(RFP) and the plasmid BBa_I739204.
- Digest BBa_I739006(cI) in BBa_I739204 with SpeI/PstI.
- Digest BBa_I739010(RFP) in BBa_I739204 with SpeI/PstI.
- Ligate digested BBa_I739006(cI) in BBa_I739204 with digested BBa_I739007(P22 cII). You get a plasmid containing a BBa_I739006(cI).BBa_I739007(P22 cII) composite part.
- Ligate digested BBa_I739010(RFP) in BBa_I739204 with BBa_I739011(GFP). You get a plasmid containing a BBa_I739010(RFP).BBa_I739011(GFP) composite part.
- Digest BBa_I739006(cI).BBa_I739007(P22 cII) with SpeI/PstI.
- Digest BBa_I739010(RFP).BBa_I739011(GFP) with XbaI/PstI.
- Ligate digested BBa_I739006(cI).BBa_I739007(P22 cII) and digested BBa_I739010(RFP).BBa_I739011(GFP). You get the completed plasmid containing the BBa_I739006(cI).BBa_I739007(P22 cII).BBa_I739010(RFP).BBa_I739011(GFP) composite part.
References
[1] Sambrook J and Russel DW "Molecular Cloning: A Laboratory Manual", Cold Spring Harbour Laboratory Press, 3rd edition, 2001
[2] Bolivar F, Rodriguez RL, Greene PJ, Betlach MC, Heynecker HL and Boyer HW "Construction of useful cloning vectors", Gene 2: 95-113, 1977
[3] Watson N "A new revision of the sequence of plasmid pBR322", Gene 70: 399-403, 1988
[4] QIAGEN FAQs
[5] Fernández S et al. "Activation of the transcriptional regulator XylR of Pseudomonas putida by release of repression between functional domains", Mol Microbiol 16(2):205-213, 1995]
[6] Chang ACY and Cohen SN "Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid", J Bacteriol 134: 1141-1156, 1978
[7] Rose, R.E., "The nucleotide sequence of pACYC177", Nucleic Acids Res, 16(1): 356, 1988
[8] Standard Assembly Process