Imperial/Wet Lab/Results/CBD1.1
From 2007.igem.org
m (→Materials and Methods) |
m (→Materials and Methods) |
||
| Line 12: | Line 12: | ||
== Materials and Methods == | == Materials and Methods == | ||
| - | Link to the [[Imperial/Wet_Lab/Protocols/CBD1|Protocol] | + | Link to the [[Imperial/Wet_Lab/Protocols/CBD1|Protocol]] |
==Results== | ==Results== | ||
Revision as of 23:47, 24 October 2007
In vivo Testing of pTet-GFP and pT7-GFP Constructs
Aims
To determine if the following constructs work in vivo:
- [http://partsregistry.org/Part:BBa_I13522 pTet-GFP]
- [http://partsregistry.org/Part:BBa_E7104 pT7-GFP]
The testing was comprised of several tests:
- pTet and pT7 in vivo - Tested 16-08-2007
- Retesting pT7 in vivo - Tested 17-08-2007
Materials and Methods
Link to the Protocol
Results
Test: 16-08-2007
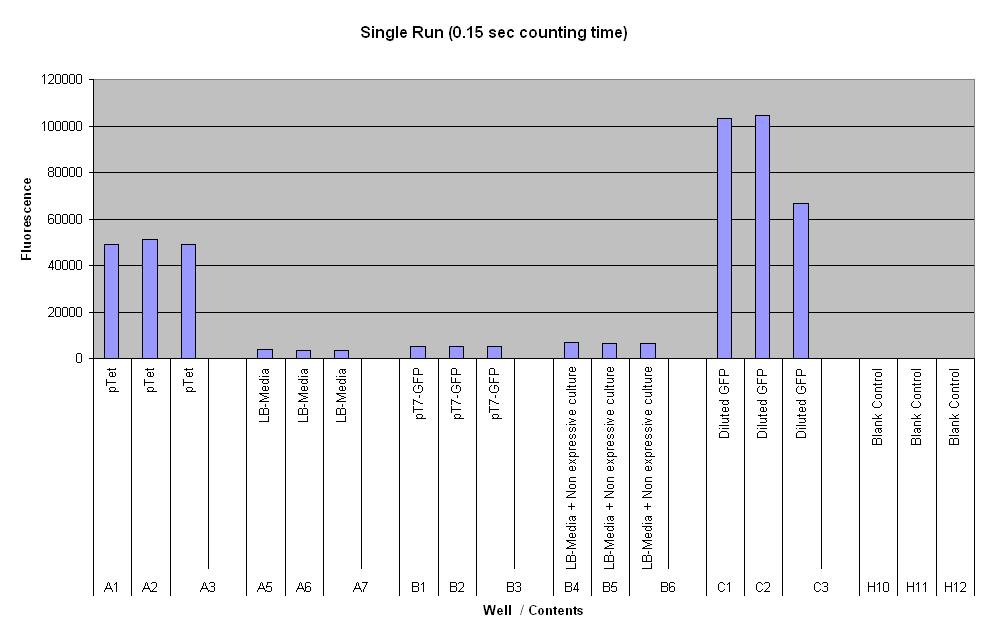
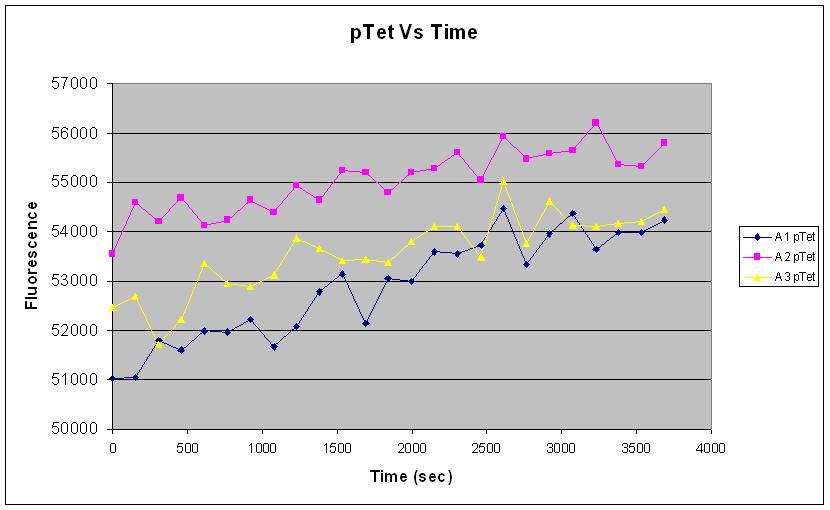
| pTet-GFP and pT7-GFP were both compared to a negative and positive control, where pTet-GFP showed significant fluorescence, while pT7 did not show any dramatic increase (Fig.1). When measured over intervals, pTet showed a steady increase in fluorescence (Fig.2). Fig.2 also suggests that there may be some variation across the samples that have been measured. |
Test: 17-08-2007
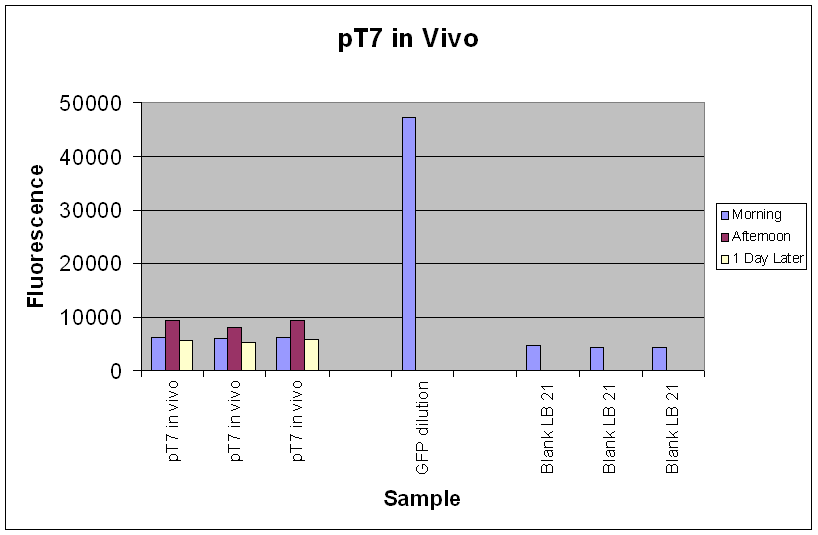
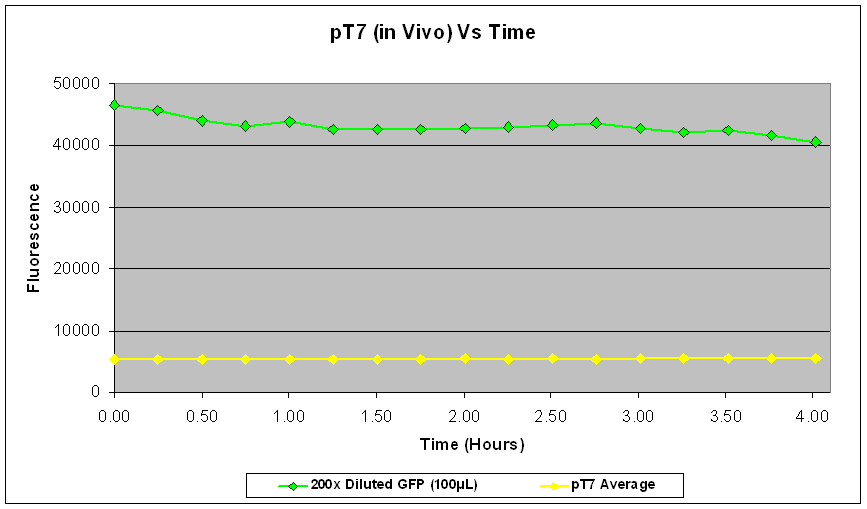
| Fig.3 shows that the pT7-GFP construct had significant fluorescence compared to the negative control, which seems to suggest that GFP has been expressed. However, when fluorescence correlated over the time period, there was little or no increase in fluorescence (Fig.4), suggesting little or no expression of GFP over the time course. |
Controls:
- Positive control - diluted GFP solution of equal volume
- Negative control - LB media of equal volume
[http://openwetware.org/images/4/42/IC2007_Experiments_Phase_1_Results-16-08-2007.xls Complete set of results and raw data ]
Discussion
[http://partsregistry.org/Part:BBa_I13522 pTet-GFP]
Fig.1 and Fig.2 both strongly indicate that there was a good amount of expression of GFP (~54000) with the pTet-GFP construct, leading to an increase in fluoresence over time. This shows that the construct is functioning well in vivo.
Fig.2 however suggests that there may be significant variation in the measurement of our samples. This could be due to experimental methodology, or the intrinsic nature of variability of expression in the in vivo chassis.
[http://partsregistry.org/Part:BBa_E7104 pT7-GFP]
As our initial tests of the pT7-GFP construct did not yield expected results, it was postualated that the absence of fluorescence increase could be due to several reasons. Firstly, the cells might not be properly induced, to which IPTG of a suitable concentration(~1mM) needs to be added, and the culture grown overnight. It could also be that the construct was not working at all, or that the culture was in stationary phase when it was tested, leading to little or not expression of GFP.
In the re-test, the culture had been properly induced with IPTG at growth phase. While absolute fluorescence levels indicate the presence of GFP in the culture, it was not as significant as that of pTet-GFP. In addition, there was little or no increase of fluorescence over the time period at which it was measured. This could be due to the fact that the pT7-GFP construct either did not express as quickly or strongly as pTet-GFP in vivo, or that the construct was simply not working. Further investigation into the construct also revealed a weaker ribosome binding site compared to the pTet-GFP construct which could account for the weak fluorescent signal.
Conclusion
We investigated the possibility of using different constructs, and strength of different promoters for our projects. To conclude,
- pTet-GFP construct gave a strong fluorescent signal, indicating good expression of GFP in vivo.
- pT7-GFP construct gave little or no fluorescent signal, to which several reasons had been suggested, and further experiments are required to corrobate them.