Melbourne/Lab BL Notebook/20070916PCR1
From 2007.igem.org
Contents |
Protocol for PCR reactions A~G
For amplifying the photoreceptor and transmembrane domains of NpSopII-NpHtrII. All -ve controls were clean.
| PCR mix | PCR program | PrimerII |
|---|---|---|
| 5ul 10x buffer\\
5ul 10x Enhancer 0.6ul dNTPs (25mM stock) 2.5ul MgSO4 (Supplied in PCR kit) 1.5ul Primer BL_FP1_s (10uM stock) 1.5ul Primer II (10uM stock) 1ul Template (pJS010) 0.4ul Pfx Platinum (Invitrogen) 32.5ul ddH2O | 94°C - 5'
94°C - 30" 59°C - 35" 68°C - 1.5' (goto step 2 x30) 68°C -10' 4°C forever | Reaction A => Primer BL_Con1_as (Some non-specific bands)
Reaction B => Primer BL_Con2_as Reaction C => Primer BL_Con3_as (Some non-specific bands) Reaction D => Primer BL_Con4_as Reaction E => Primer BL_Con5_as Reaction F => Primer BL_Con6_as Reaction G => Primer BL_Con7_as |
| 50ul Total |
Protocol for PCR reactions 1-7
For amplification of the kinase domain of ComP
| PCR mix | PCR program | PrimerI |
|---|---|---|
| 5ul 10x buffer\\
2.5ul MgSO4 (Supplied in PCR kit) 1.5ul Primer I (10uM stock) 1.5ul Primer VR (10uM stock) 1ul Template (pJS010) 0.4ul Pfx 32.5ul ddH2O | 94°C - 5'
94°C - 30" 59°C - 35" 68°C - 1.5' (goto step 2 x30) 68°C - 10' 4°C forever | Reaction 1 => Primer BL_Con1_s
Reaction 2 => Primer BL_Con2_s Reaction 3 => Primer BL_Con3_s Reaction 4 => Primer BL_Con4_s Reaction 5 => Primer BL_Con5_s Reaction 6 => Primer BL_Con6_s Reaction 7 => Primer BL_Con7_s |
| 50ul Total |
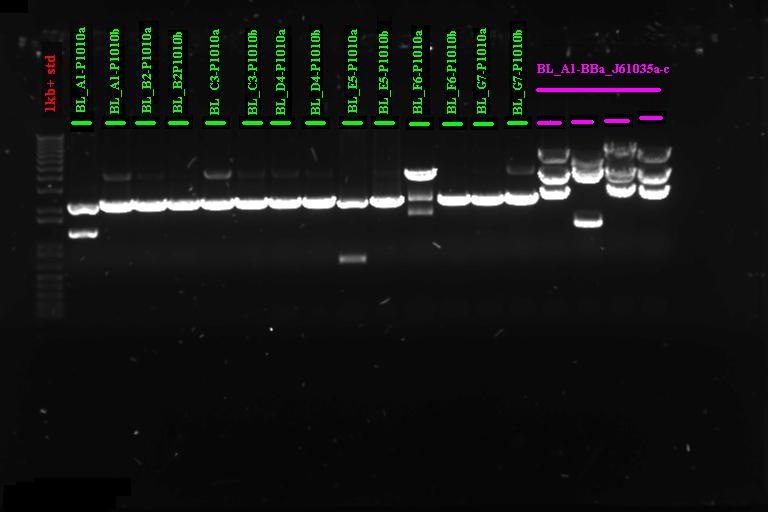
Gel Purification of PCR products A~G and 1~7
PCR product of the expected size was excised from .8% agarose gel and gel purified using the Invitrogen gel purification kit. Protocol as detailed in the kit.
Second Round PCR
For stitching products A~G to 1~7. Gel purified PCR products from the above reaction were used.
| PCR mix | PCR program | <Reaction, TemplateI, TemplateII> |
|---|---|---|
| 5ul 10x buffer\\
2.5ul 10x Enhancer 0.6ul dNTPs (25mM stock) 2.5ul MgSO4 (Supplied in PCR kit) 1.5ul Primer BL_FP1_s (10uM stock) 1.5ul Primer VR (10uM stock) 5ul Template I {A~G} 5ul Template II {1-7} 0.4ul Pfx 27ul ddH2O | 94°C - 5'
94°C - 1' 50°C - 1' 68°C - 3' (goto step 2 x30) 68°C - 10' 4°C forever |
|
| 50ul Total |
Gel purification of second round PCR product
All the reactions had large smears, but we cut out a band around 2.3kb (expected size) and gel purified as above.
Digestion/Ligation of Second Round PCR product
Per PCR reaction:
| Per 2° PCR {A1~G7} |
|---|
| 6ul purified DNA {A1~G7}
2ul 10x NEB Buffer 2 2ul 10x BSA 1ul XbaI 1ul PstI 8ul H2O |
| 20ul Total |
Also...
| BBa_J61035 (S/P) |
|---|
| 5ul [http://partsregistry.org/Part:BBa_J61035 BBa_J61035]
2ul 10x NEB Buffer 2 2ul 10x BSA 1ul SpeI 1ul PstI 9ul H2O |
| 20ul Total |
And...
| BBa_P1010 (X/P) |
|---|
| 5ul [http://partsregistry.org/Part:BBa_P1010 BBa_P1010](AmpR)
2ul 10x NEB Buffer 2 2ul 10x BSA 1ul XbaI 1ul PstI 9ul H2O |
| 20ul Total |
The latter two reactions were gel purified and the following ligations were set up:
Ligation of constructs into Death/RBS plasmid
| Per 2° PCR {A1~G7} |
|---|
| 5ul X/P digested DNA {A1~G7}
4ul X/P digested BBa_P1010 10ul 2x Quick ligase buffer 1ul T4 Ligase |
| 20ul Total |
| Per 2° PCR {A1~G7} |
|---|
| 5ul X/P digested DNA {A1~G7}
4ul S/P digested BBa_J61035 10ul 2x Quick ligase buffer 1ul T4 Ligase |
| 20ul Total |
Incubated at RT for 2hrs then transformed into competent DH5-alpha. Also included some 5minute ligation incubation controls => 5 minute ligations are significantly (~5x) more efficient than 2 hours.
Pick 2 colonies per death plasmid ligation, and 4 colonies per RBS ligation and culture in LB+Amp for 12hrs.
Miniprep (nuclease free water elution)
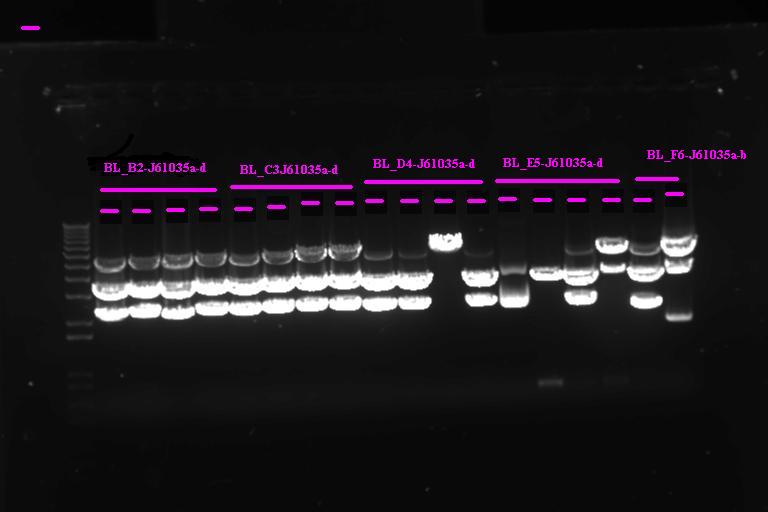
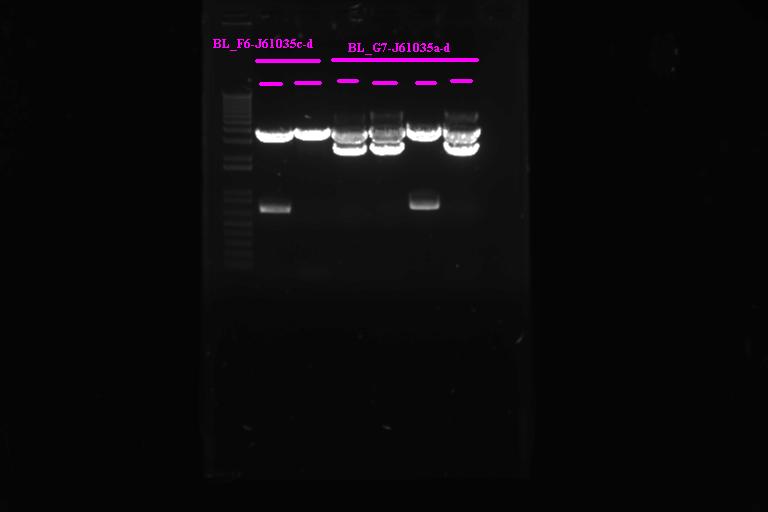
Confirmation Digest
| Per miniprepped sample |
|---|
| 5ul miniprepped sample
1ul 10x NEB Buffer 2 1ul 10x BSA .17ul XbaI .17ul PstI 2.5ul H2O |
| 20ul Total |