Tokyo/Works
From 2007.igem.org
Abstract Concept & Model Requirements Genetic_circuit Works About_our_team
0. Hybrid promoter 1. Formulation 2. Assay1 3. Simulation 4. Assay2 5. Future works
How to reach our goal
We have alternatly implemented Wet and Dry experiments to achieve our goal
To establish our “balanced-differentiation” model, genetic circuit was developed. We have drawn a navigational chart on our project by Dry approaches, which has been confirmed and reinforced by the data from Wet approaches. In short, we have alternately conducted successive combinations of Dry and Wet approaches.
0. Wet : Hybrid promoter
First, the newly devised promoter sensed the Two inputs is necessary for the Genetic_circuit.
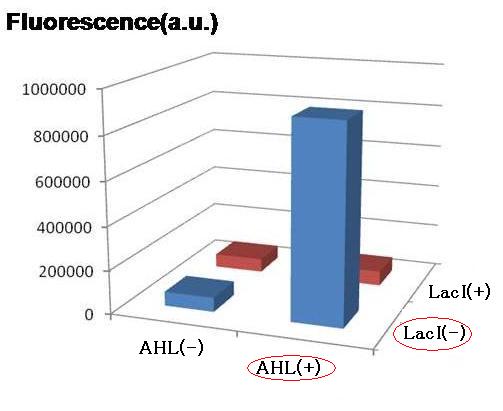
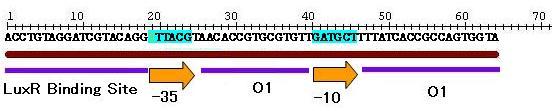
For initial attempts at regulating differentiation of E.coli by a set of two inputs, new hybrid promoter was designed . There is no desirable part among previous publications as well as BioBrick parts. Thus, we designed the parts that can be activated by LuxR and repressed by LacI . (fig.1).These brand-new parts worked as designed (fig.2).(See more.)
1.Dry : Formulation
Second, we have tried to mathmatically express our model.
From simple equations, the equations necessary for our model have been deviated step by step. Though a parameter for production rate of AHL changes cell behavior from conventional mutual-inhibition model, Hill coefficients for the promoters are still critical for emergence of bistability.(See more.)
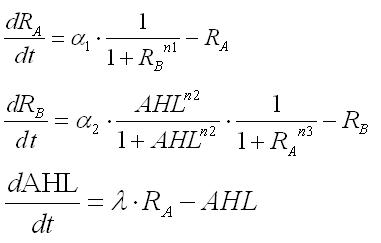
2.Wet : Assay1 using "Externally added" materials
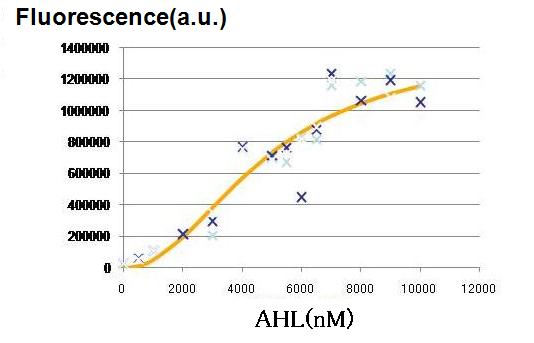
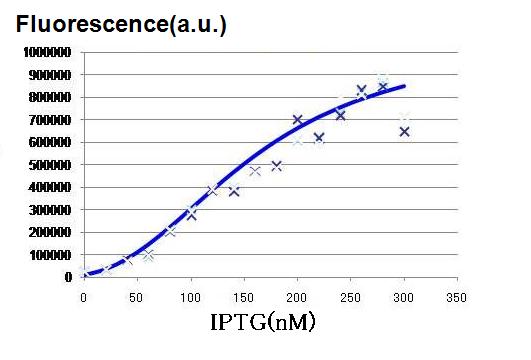
Though Hill coefficients are critical for the differentiation, adjustments of Hill coefficients by DNA sequence modification are much more difficult than those of production rate parameters; changes in RBS -35 box, or -10 box allow production efficiency. In order to confirm feasibility of our model, we focused to measure Hill coefficients of our new promoter part.(See more.)
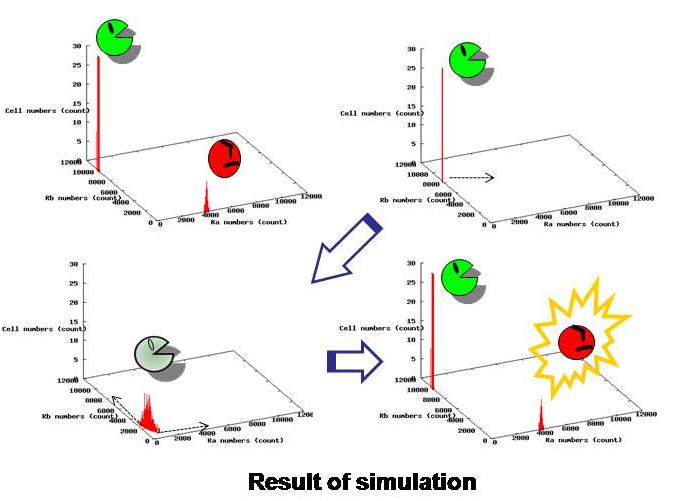
3.Dry : Simulation
Using the data from actual experiments, we simulated behaviors of our model.
Based on stochastic simulation using parameters obtained from our wet experiments, we have confirmed that the whole system becomes unstable when there are only idlers left, and then, they become either of workers and idlers. Also we have determined other parameters necessary for the desired behavior of this system. (See more.)
4.Wet : Assay2 on "Cell-produced" AHL and Expression comparison
Here the simulation results were tested by actual wet experiments.
Whether the parameters obtained in 3 is feasible in actual Wet experiments was tested by focusing on the amount of cell-produced AHL as well as the strength of the promoters. Indication from the result is applied for the next work.(See more.)
5.Future works
Through the experiments mentioned above, we have found that our model can be completed by changing some of the parts employed so far. Continuing such simulation oriented construction of genetic circuit, it will be increasingly necessary to exchange parts. Here we would like to offer useful means for part exchanges.
Based on the results from wet experiments, the next dry approach - analysis and simulation - should be performed, which will in turn be testified and confirmed by wet experiments. Continueing these processes, if time permits, would further sophisticate and complete our model. (See more.)