|
Introduction: Bacteriophage Lambda
Lambda phage is a well-known and extensively used vector in molecular biology. Lambda is a bacteria virus that can take two different pathways after entering a cell:
- The most common pathway is the lytic pathway. Upon entering the cell, the virus commands the
cellular machinery to make new phage particles and, within about 45 minutes, lyses the cell.
- Alternatively, lambda can enter the lysogenic pathway. The viral genome is incorporated into the cell's chromosomal DNA and remains dormant until the right conditions arise (usually involving cellular stress).
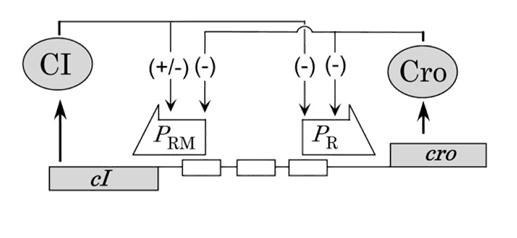
Lambda's decision is controlled by the lambda promoter. If enough cI is produced first, the cell becomes lysogenic. If Cro predominates, the cell goes lytic and bursts.
Updates
First Progress Update
July 11, 2007
Since no one in our lab had worked with phage before, being able to create and use the lysates has been a learning experience. We have relied heavily on Tim Larson, a faculty adviser from Biology, to help us work through the issues we've come across. We are currently working with two types of phage: Lambda ATCC, a wild-type phage, and Lambda EYFP, a fluorescent phage. Here is what we've accomplished so far with each:
Lambda ATCC:
- 1) Create a high-titer lysate.
- 2) Infect plates of all 3 strains of E. coli we are working with: LE392, ATCC C600, and a NEB strain. All 3 form plaques approximately equally.
- 3) View lysis in liquid cultures.
- 4) Pending: Examine how liquid cultures behave with different amounts of phage using a microtiter plate reader which takes OD readings over time.
Lambda EYFP:
- 1) Induce NM538 lysogens and create a lysate.
- 2) Infect plates of all 3 strains of bacteria we are working with: LE392, ATCC C600, and a NEB strain. The LE392 plate formed plaques at higher dilutions than did either the C600 or NEB plates, which performed the same.
- 3) Pending: Understand why the LE392 plate performed differently.
- 4) Pending: View the fluorescence, if possible, using microscopy.
|

