Glasgow/Wetlab/Gels
From 2007.igem.org
(→Gels) |
|||
| (48 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | [[Glasgow|Glasgow Main Page]] | + | {| valign=top cellpadding=3 |
| + | |- | ||
| + | !align=center|[[Image:Uog.jpg]] || [[Glasgow|<font face=georgia color=#3366CC size=4>Back To <br> Glasgow's <br> Main Page</font>]] || [[Glasgow/Wetlab|<font face=georgia color=#3366CC size=4>Back To <br> Glasgow's <br> Wetlab Log</font>]] | ||
| + | |} | ||
---- | ---- | ||
| - | + | = Gels = | |
| - | + | ||
| + | ==Friday 10th August 2007== | ||
| + | |||
{| border="1" cellspacing="0" cellpadding="5" align="center" | {| border="1" cellspacing="0" cellpadding="5" align="center" | ||
!Date and Reference | !Date and Reference | ||
| Line 9: | Line 14: | ||
!Gel | !Gel | ||
|- | |- | ||
| - | + | |[[Glasgow/Wetlab/Week6#Friday 10th August 2007|10th Aug 2007 (2)]] | |
| - | + | |Colony PCR after ligating phzM, phzS, DntR and Pu with the construction vectors 3/20G and 4/6B. Gel 1. | |
| - | + | |'''1.'''phzM<br> | |
| - | + | '''2.'''phzM<br> | |
| - | + | '''3.'''phzS<br> | |
| - | + | '''4.'''phzS<br> | |
| - | + | '''5.'''phzS<br> | |
| - | + | '''6.'''phzS<br> | |
| - | + | '''7.'''neg control<br> | |
| - | + | '''8.'''neg control<br> | |
| - | |[[Glasgow/Wetlab/Week6#Friday 10th August 2007| | + | '''9.'''neg control<br> |
| - | |Colony PCR after ligating | + | |
| - | | | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
|[[Image:10thAug gel 1.JPG|200px]] | |[[Image:10thAug gel 1.JPG|200px]] | ||
|- | |- | ||
| - | |[[Glasgow/Wetlab/Week6#Friday 10th August 2007| | + | |[[Glasgow/Wetlab/Week6#Friday 10th August 2007|10th Aug 2007 (2)]] |
| - | |Colony PCR after ligating | + | |Colony PCR after ligating phzM, phzS, DntR and Pu with the construction vectors 3/20G and 4/6B. Gel 2. |
| - | | | + | |'''1.'''phzS<br> |
| - | + | '''2.'''phzS<br> | |
| - | + | '''3.'''neg control<br> | |
| - | + | '''4.'''DntR<br> | |
| - | + | '''5.'''DntR<br> | |
| - | + | '''6.'''Pu<br> | |
| - | + | '''7.'''Pu<br> | |
| - | + | '''8.'''neg control<br> | |
| - | + | '''9.'''neg control<br> | |
| - | + | '''10.'''neg control<br> | |
| - | + | '''11.'''neg control<br> | |
|[[Image:10thAug gel 2.JPG|200px]] | |[[Image:10thAug gel 2.JPG|200px]] | ||
| + | |} | ||
| + | |||
| + | ==Monday 13th August 2007== | ||
| + | |||
| + | {| border="1" cellspacing="0" cellpadding="5" align="center" | ||
| + | !Date and Reference | ||
| + | !Description | ||
| + | !Key | ||
| + | !Gel | ||
| + | |- | ||
| + | |[[Glasgow/Wetlab/Week7#Monday 13th August 2007|13th Aug 2007 (4)]] | ||
| + | |PCR of genes phzA→phzG, phzA→phzD, and phzD→phzG. Wells in the gel were made larger by putting tape on the combs in order to yield more DNA in gel extraction. | ||
| + | |'''1A.'''phzA→phzG <br> | ||
| + | '''1B.'''phzA→phzG <br> | ||
| + | '''1C.'''phzA→phzG <br> | ||
| + | '''2A.'''phzA→phzG <br> | ||
| + | '''2B.'''phzA→phzD <br> | ||
| + | '''2C.'''phzD→phzG <br> | ||
| + | |[[Image:2007-08-13 12hr 43min.JPG|220px]] | ||
| + | |} | ||
| + | |||
| + | ==Friday 17th August 2007== | ||
| + | |||
| + | {| border="1" cellspacing="0" cellpadding="5" align="center" | ||
| + | !Date and Reference | ||
| + | !Description | ||
| + | !Key | ||
| + | !Gel | ||
| + | |- | ||
| + | |[[Glasgow/Wetlab/Week7#Friday 17th August 2007|17th Aug 2007 (2-4)]] | ||
| + | |PstI digested minipreps of phzM and phzS in 4/6B and 3/20G vectors before and after the first round of site-directed mutagenesis. Gel 1. | ||
| + | |'''1.-3.'''phzM+4/6B<br>(PstI site mutated)<br> | ||
| + | '''4.-6.'''phzM+3/20G<br>(PstI site mutated)<br> | ||
| + | '''7.-9.'''phzS+4/6B<br>(1st PstI site mutated)<br> | ||
| + | '''10.-12.'''phzS+3/20G<br>(2nd PstI site mutated)<br> | ||
| + | '''13.-15.'''phzS+4/6B<br>(2nd PstI site mutated)<br> | ||
| + | '''16.'''phzS+3/20G<br>(1st PstI site mutated)<br> | ||
| + | |[[Image:17thAug SDM1 PstI digest gel 1.JPG|220px]] | ||
| + | |- | ||
| + | |[[Glasgow/Wetlab/Week7#Friday 17th August 2007|17th Aug 2007 (2-4)]] | ||
| + | |PstI digested minipreps of phzM and phzS in 4/6B and 3/20G vectors before and after the first round of site-directed mutagenesis. Gel 2. | ||
| + | |'''1.-2.'''phzS+3/20G<br>(1st PstI site mutated)<br> | ||
| + | '''3.'''phzM+4/6B<br>('''Before''' mutagenesis)<br> | ||
| + | '''4.'''phzM+3/20G<br>('''Before''' mutagenesis)<br> | ||
| + | '''5.'''phzS+4/6B<br>('''Before''' mutagenesis)<br> | ||
| + | '''6.'''phzS+3/20G<br>('''Before''' mutagenesis)<br> | ||
| + | |[[Image:17thAug SDM1 PstI digest gel 2.JPG|220px]] | ||
| + | |} | ||
| + | |||
| + | ==Thursday 23rd August 2007== | ||
| + | |||
| + | {| border="1" cellspacing="0" cellpadding="5" align="center" | ||
| + | !Date and Reference | ||
| + | !Description | ||
| + | !Key | ||
| + | !Gel | ||
| + | |- | ||
| + | |[[Glasgow/Wetlab/Week8#Thursday 23rd August 2007|23rd Aug 2007]] | ||
| + | |phzS minipreps in 4/6B and 3/20G run after the second round of site-directed mutagenesis. | ||
| + | |'''1.-5.'''phzS+4/6B<br> | ||
| + | '''6.-16.'''phzS+3/20G<br> | ||
| + | |[[Image:Maija's Gel taken by Scott.JPG|200px]] | ||
| + | |- | ||
| + | |[[Glasgow/Wetlab/Week8#Thursday 23rd August 2007|23rd Aug 2007]] | ||
| + | |PstI digested phzS minipreps in 4/6B and 3/20G vectors after the second round of site-directed mutagenesis. | ||
| + | |'''1.-5.'''phzS+4/6B<br> | ||
| + | '''6.-16.'''phzS+3/20G<br> | ||
| + | |[[Image:23rdAug 13hr 46min Maija.JPG|200px]] | ||
| + | |- | ||
| + | |[[Glasgow/Wetlab/Week8#Thursday 23rd August 2007|23rd Aug 2007]] | ||
| + | |Colony PCR with "Pr_Prefix" and "XylR_Suffix" primers from Lynsey's transformations with Pr+XylR miniprep DNA. Gel 1. | ||
| + | |'''1.-7.'''colonies from the plate | ||
| + | |[[Image:Gel for Maija 1.JPG|200px]] | ||
| + | |- | ||
| + | |[[Glasgow/Wetlab/Week8#Thursday 23rd August 2007|23rd Aug 2007]] | ||
| + | |Colony PCR with "Pr_Prefix" and "XylR_Suffix" primers from Lynsey's transformations with Pr+XylR miniprep DNA. Gel 2. | ||
| + | |'''8.-12.'''colonies from the plate<br> | ||
| + | '''13.'''neg control | ||
| + | |[[Image:Gel for Maija 2.JPG|200px]] | ||
|} | |} | ||
<br> | <br> | ||
| + | |||
| + | ==Friday 24th August 2007== | ||
| + | |||
| + | {| border="1" cellspacing="0" cellpadding="5" align="center" | ||
| + | !Date and Reference | ||
| + | !Description | ||
| + | !Key | ||
| + | !Gel | ||
| + | |- | ||
| + | |[[Glasgow/Wetlab/Week8#Friday 24th August 2007|24th Aug 2007 (1)]] | ||
| + | |These are the miniprepped plasmids from Pr+XylR in TOPO vector. The seven colonies gave the right size product from colony PCR but failed to give right size bands after miniprepping. Wait for sequencing results. | ||
| + | |'''1.-7.'''colonies from Pr+XylR in TOPO transformations | ||
| + | |[[Image:2007-08-24 12hr 54min Maija.JPG|200px]] | ||
| + | |} | ||
| + | |||
| + | ==Monday 27th August 2007== | ||
| + | |||
| + | {| border="1" cellspacing="0" cellpadding="5" align="center" | ||
| + | !Date and Reference | ||
| + | !Description | ||
| + | !Key | ||
| + | !Gel | ||
| + | |- | ||
| + | |[[Glasgow/Wetlab/Week9#Monday 27th August 2007|27th Aug 2007 (1)]] | ||
| + | |pDntA in 3/20G and 4/6B vectors digested with Roche's PvuI restriction enzyme. Results do not match up with expected fragment sizes, thus performing a PCR with "VF2" and "VR" primers. | ||
| + | |'''1.'''pDntA+3/20G<br> | ||
| + | '''2.'''pDntA+4/6B | ||
| + | |[[Image:2007-08-27 13hr 21min Maija.JPG|170px]] | ||
| + | |} | ||
| + | ---- | ||
| + | {| valign=top cellpadding=3 | ||
| + | |- | ||
| + | !align=center|[[Image:Uog.jpg]] || [[Glasgow|<font face=georgia color=#3366CC size=4>Back To <br> Glasgow's <br> Main Page</font>]] || [[Glasgow/Wetlab|<font face=georgia color=#3366CC size=4>Back To <br> Glasgow's <br> Wetlab Log</font>]] | ||
| + | |} | ||
Latest revision as of 17:06, 22 October 2007
 | Back To Glasgow's Main Page | Back To Glasgow's Wetlab Log |
|---|
Contents |
Gels
Friday 10th August 2007
| Date and Reference | Description | Key | Gel |
|---|---|---|---|
| 10th Aug 2007 (2) | Colony PCR after ligating phzM, phzS, DntR and Pu with the construction vectors 3/20G and 4/6B. Gel 1. | 1.phzM 2.phzM | 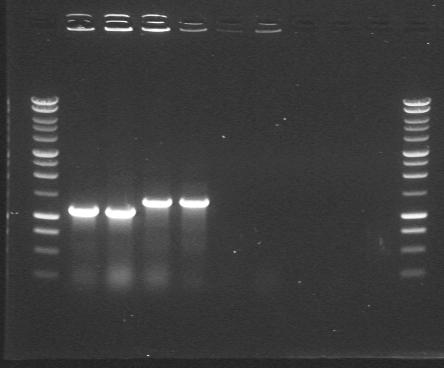
|
| 10th Aug 2007 (2) | Colony PCR after ligating phzM, phzS, DntR and Pu with the construction vectors 3/20G and 4/6B. Gel 2. | 1.phzS 2.phzS | 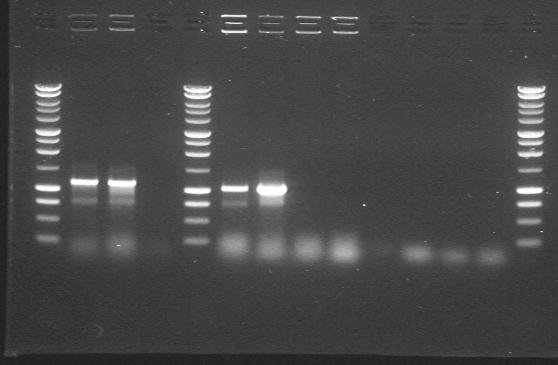
|
Monday 13th August 2007
| Date and Reference | Description | Key | Gel |
|---|---|---|---|
| 13th Aug 2007 (4) | PCR of genes phzA→phzG, phzA→phzD, and phzD→phzG. Wells in the gel were made larger by putting tape on the combs in order to yield more DNA in gel extraction. | 1A.phzA→phzG 1B.phzA→phzG | 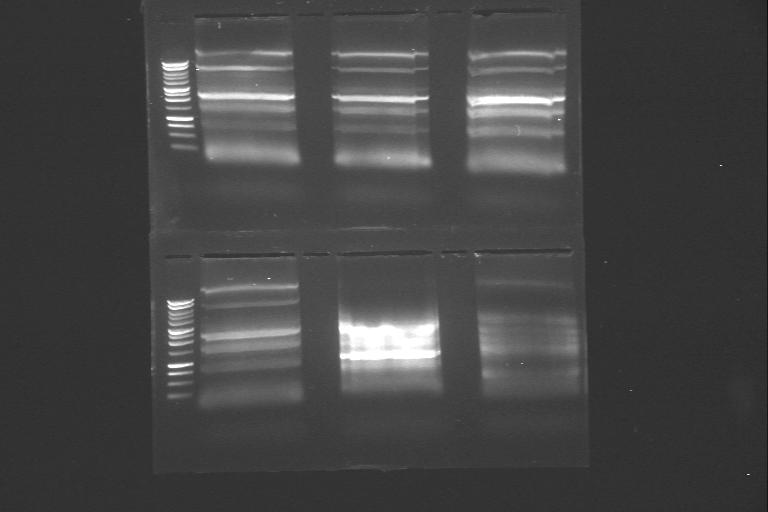
|
Friday 17th August 2007
| Date and Reference | Description | Key | Gel |
|---|---|---|---|
| 17th Aug 2007 (2-4) | PstI digested minipreps of phzM and phzS in 4/6B and 3/20G vectors before and after the first round of site-directed mutagenesis. Gel 1. | 1.-3.phzM+4/6B (PstI site mutated) 4.-6.phzM+3/20G | 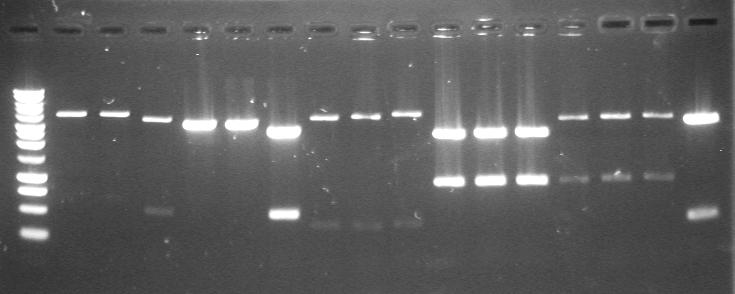
|
| 17th Aug 2007 (2-4) | PstI digested minipreps of phzM and phzS in 4/6B and 3/20G vectors before and after the first round of site-directed mutagenesis. Gel 2. | 1.-2.phzS+3/20G (1st PstI site mutated) 3.phzM+4/6B | 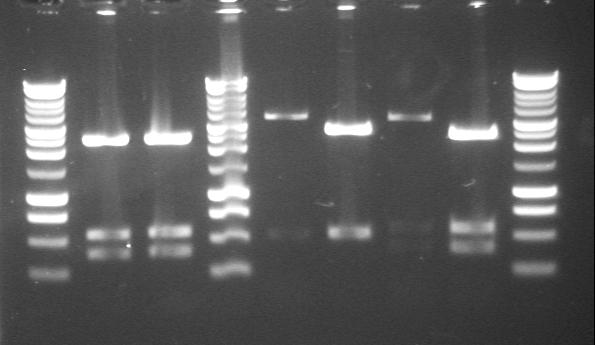
|
Thursday 23rd August 2007
| Date and Reference | Description | Key | Gel |
|---|---|---|---|
| 23rd Aug 2007 | phzS minipreps in 4/6B and 3/20G run after the second round of site-directed mutagenesis. | 1.-5.phzS+4/6B 6.-16.phzS+3/20G | 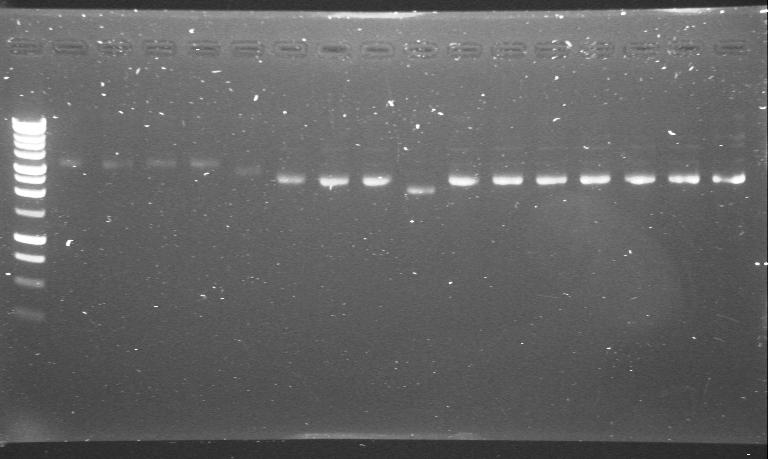
|
| 23rd Aug 2007 | PstI digested phzS minipreps in 4/6B and 3/20G vectors after the second round of site-directed mutagenesis. | 1.-5.phzS+4/6B 6.-16.phzS+3/20G | 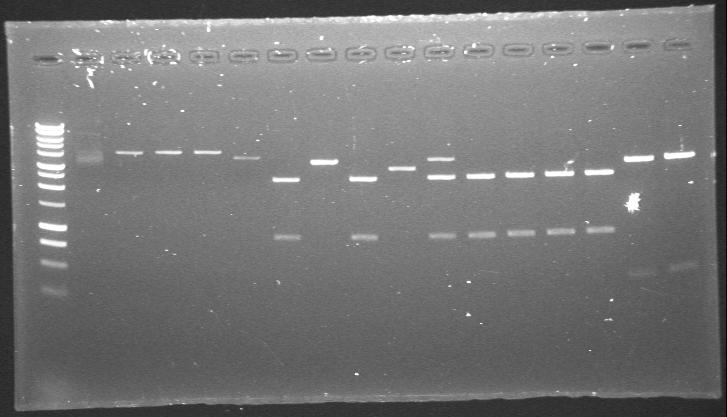
|
| 23rd Aug 2007 | Colony PCR with "Pr_Prefix" and "XylR_Suffix" primers from Lynsey's transformations with Pr+XylR miniprep DNA. Gel 1. | 1.-7.colonies from the plate | 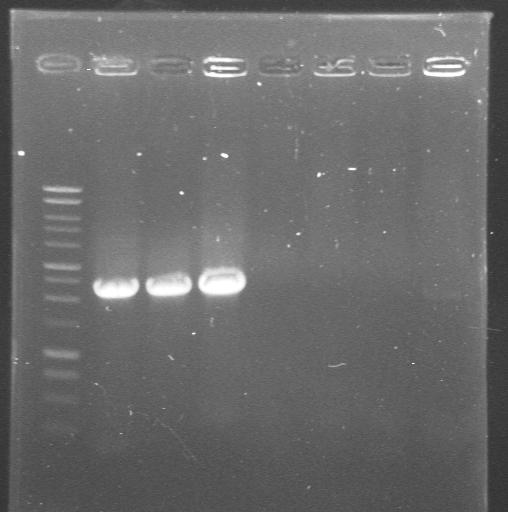
|
| 23rd Aug 2007 | Colony PCR with "Pr_Prefix" and "XylR_Suffix" primers from Lynsey's transformations with Pr+XylR miniprep DNA. Gel 2. | 8.-12.colonies from the plate 13.neg control | 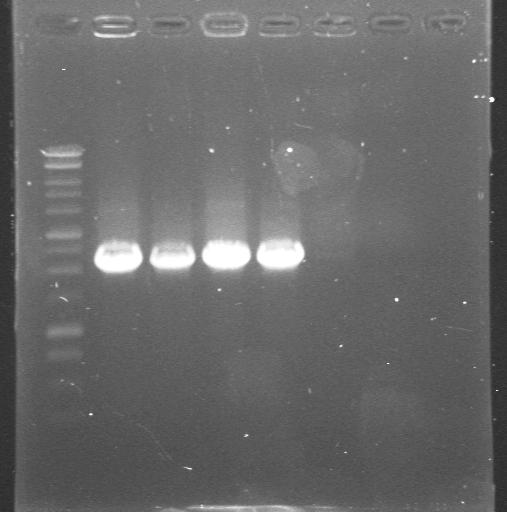
|
Friday 24th August 2007
| Date and Reference | Description | Key | Gel |
|---|---|---|---|
| 24th Aug 2007 (1) | These are the miniprepped plasmids from Pr+XylR in TOPO vector. The seven colonies gave the right size product from colony PCR but failed to give right size bands after miniprepping. Wait for sequencing results. | 1.-7.colonies from Pr+XylR in TOPO transformations | 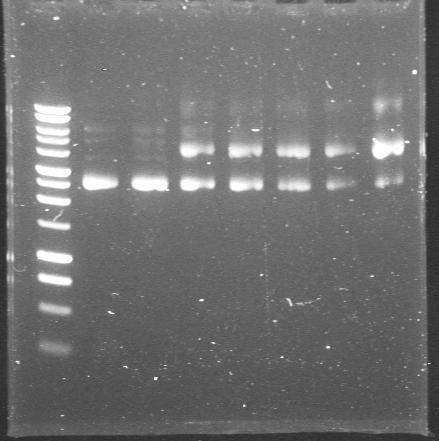
|
Monday 27th August 2007
| Date and Reference | Description | Key | Gel |
|---|---|---|---|
| 27th Aug 2007 (1) | pDntA in 3/20G and 4/6B vectors digested with Roche's PvuI restriction enzyme. Results do not match up with expected fragment sizes, thus performing a PCR with "VF2" and "VR" primers. | 1.pDntA+3/20G 2.pDntA+4/6B | 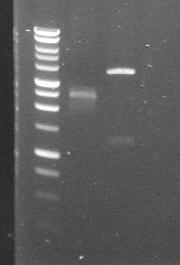
|
 | Back To Glasgow's Main Page | Back To Glasgow's Wetlab Log |
|---|