Edinburgh/Yoghurt/Proof of concept
From 2007.igem.org
(→pTG262 expression in ''Bacillus subtilis'') |
|||
| Line 7: | Line 7: | ||
In order to test the feasibility of gene expression in ''Lactobacillus'' and the possibility of making self flavouring yoghurt, we required a proof of concept. | In order to test the feasibility of gene expression in ''Lactobacillus'' and the possibility of making self flavouring yoghurt, we required a proof of concept. | ||
| - | As both the pigment and flavour pathways are rather complex and will require modification and optimisation before their expression in yoghurt we | + | As both the pigment and flavour pathways are rather complex and will require modification and optimisation before their expression in yoghurt we planned to use the much simpler Red Fluorescent Protein (RFP) gene as a proof of concept. |
| - | So far we have managed to transform a number of RFP-pTG262 vectors containing a variety of promoters into ''Bacillus subtillis'', a gram positive bacterium. | + | So far we have managed to transform a number of RFP-pTG262 vectors containing a variety of promoters into ''E. coli'' and also ''Bacillus subtillis'', a gram positive bacterium. |
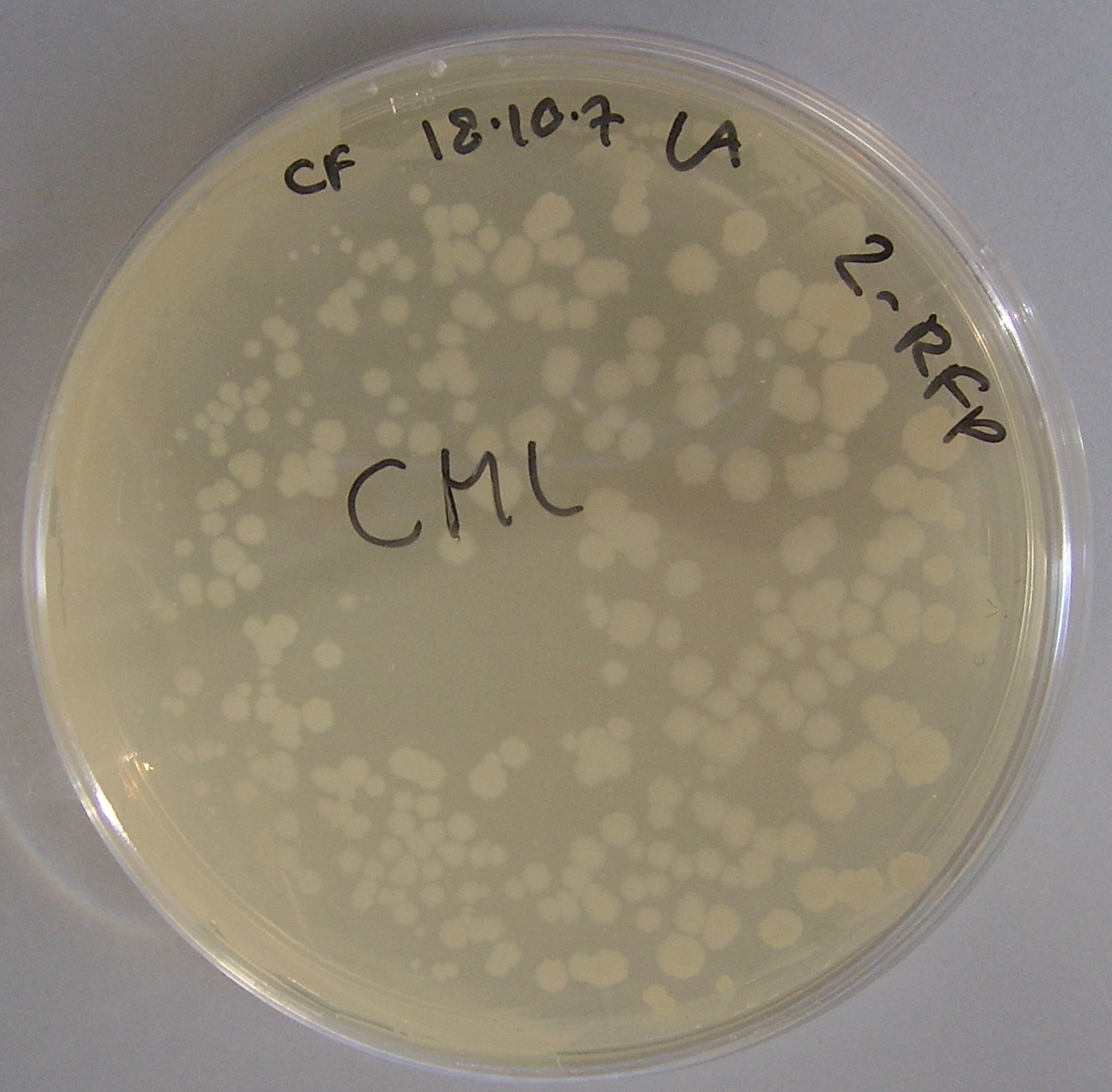
[[Image:PLac-RFP-pTG262 Bacillus.JPG|thumb|200 px|'''Fig. 1:''' ''E. coli'' which has been successfully transformed with the pTG262-pLac-RFP construct]] | [[Image:PLac-RFP-pTG262 Bacillus.JPG|thumb|200 px|'''Fig. 1:''' ''E. coli'' which has been successfully transformed with the pTG262-pLac-RFP construct]] | ||
| Line 16: | Line 16: | ||
We chose to initially transform ''Bacillus subtillis'' with our proof of concept vectors over ''Lactobacillus'' for two reasons: | We chose to initially transform ''Bacillus subtillis'' with our proof of concept vectors over ''Lactobacillus'' for two reasons: | ||
| - | 1. the bacterium has a much simpler transformation process | + | 1. the bacterium is naturally competent at certain stages of its life cycle and therefore has a much simpler transformation process |
| - | 2. members of the French lab have had | + | 2. members of the French lab have had successful results from when they previously worked with ''Bacillus subtilis'' |
| Line 26: | Line 26: | ||
We have inserted the lactose induced RFP gene into the pTG262 vector | We have inserted the lactose induced RFP gene into the pTG262 vector | ||
| - | * this vector was then transformed into E. coli | + | * this vector was then transformed into ''E. coli'' |
* growth on IPTG-Xgal media resulted in RFP expression and production of red colonies, these can be viewed in figure 1 | * growth on IPTG-Xgal media resulted in RFP expression and production of red colonies, these can be viewed in figure 1 | ||
| Line 35: | Line 35: | ||
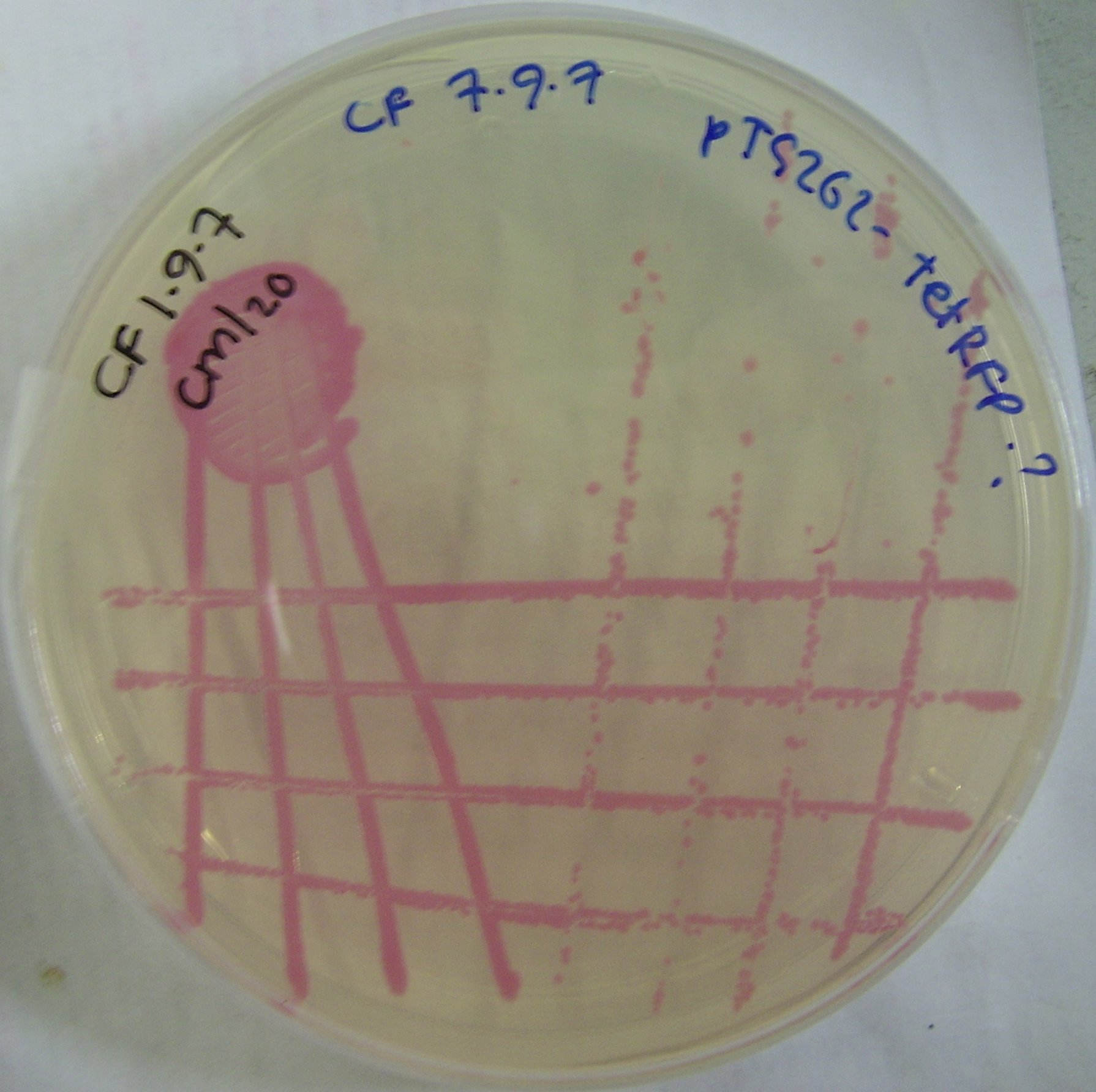
We ligated the Ptet induced RFP biobrick into the pTG262 vector. We then transformed the vector into ''E. coli''. | We ligated the Ptet induced RFP biobrick into the pTG262 vector. We then transformed the vector into ''E. coli''. | ||
| - | RFP synthesis was observed | + | RFP synthesis was observed. |
ptet incuded RFP ''E. coli'' colonies can be viewed in figure 2. | ptet incuded RFP ''E. coli'' colonies can be viewed in figure 2. | ||
| Line 43: | Line 43: | ||
==pTG262 expression in ''Bacillus subtilis''== | ==pTG262 expression in ''Bacillus subtilis''== | ||
| - | After the successful expression of both the pTG262 RFP constructs in ''E. coli'', we decided to determine if pTG262 could be stably | + | After the successful expression of both the pTG262 RFP constructs in ''E. coli'', we decided to determine if pTG262 could be stably maintained in ''Bacillus subtilis''. |
To do this we transformed both the pTG262 and Plac-RFP-pTG262 vectors into ''Bacillus subtilis''. | To do this we transformed both the pTG262 and Plac-RFP-pTG262 vectors into ''Bacillus subtilis''. | ||
| Line 51: | Line 51: | ||
This is determined by the ability of ''Bacillus'' to grow on chloramphenicol plates. | This is determined by the ability of ''Bacillus'' to grow on chloramphenicol plates. | ||
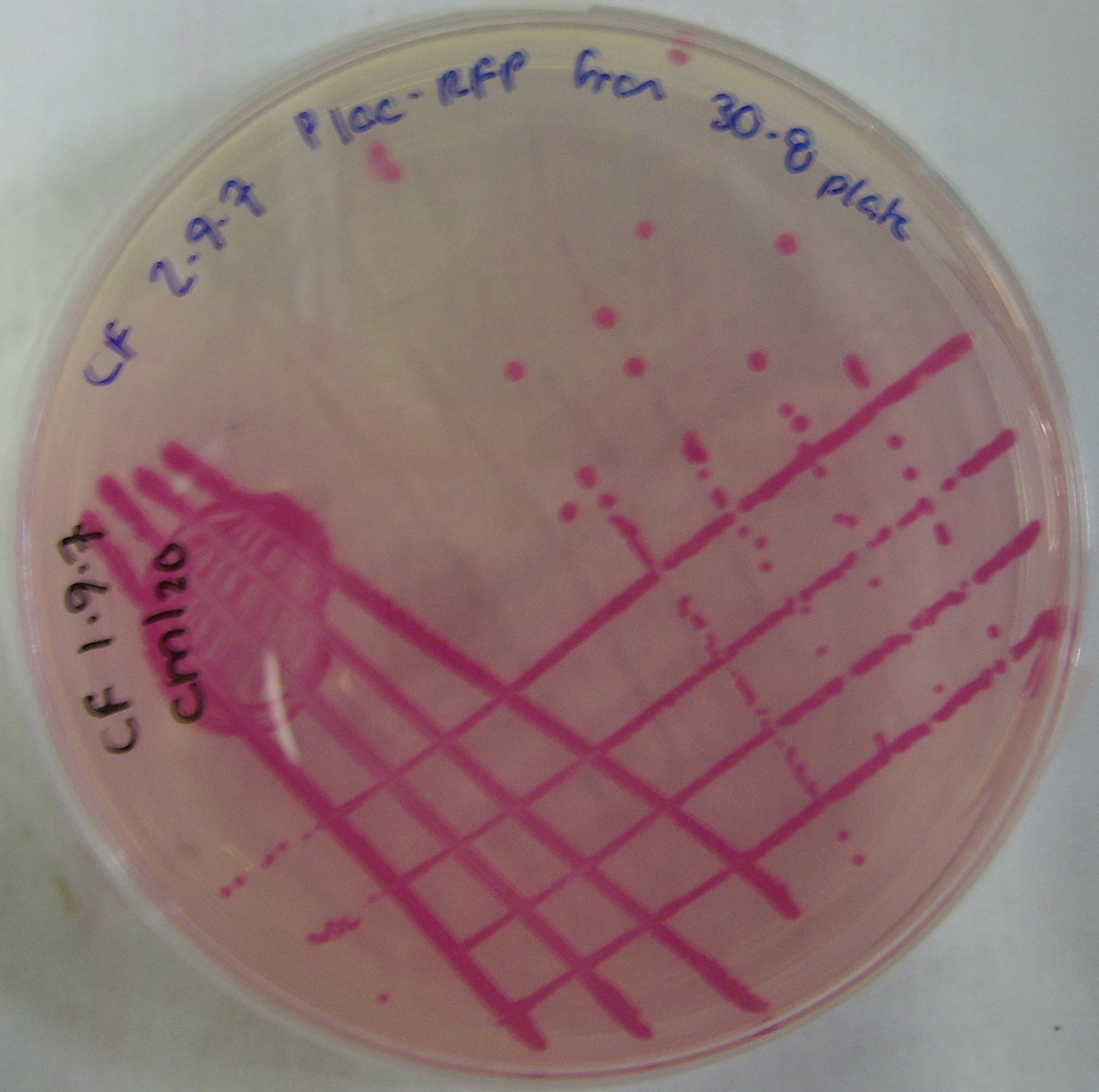
| - | Unfortunately RFP was not expressed from the pLac promoter, as shown by the colonies being white not red. We are attributing this to the ''Bacillus'' transcription machinary ( | + | Unfortunately RFP was not expressed from the pLac promoter, as shown by the colonies being white not red. We are attributing this to the ''Bacillus'' transcription machinary (particularly RNA polymerase and its associated sigma factors) not recognising the promoter. |
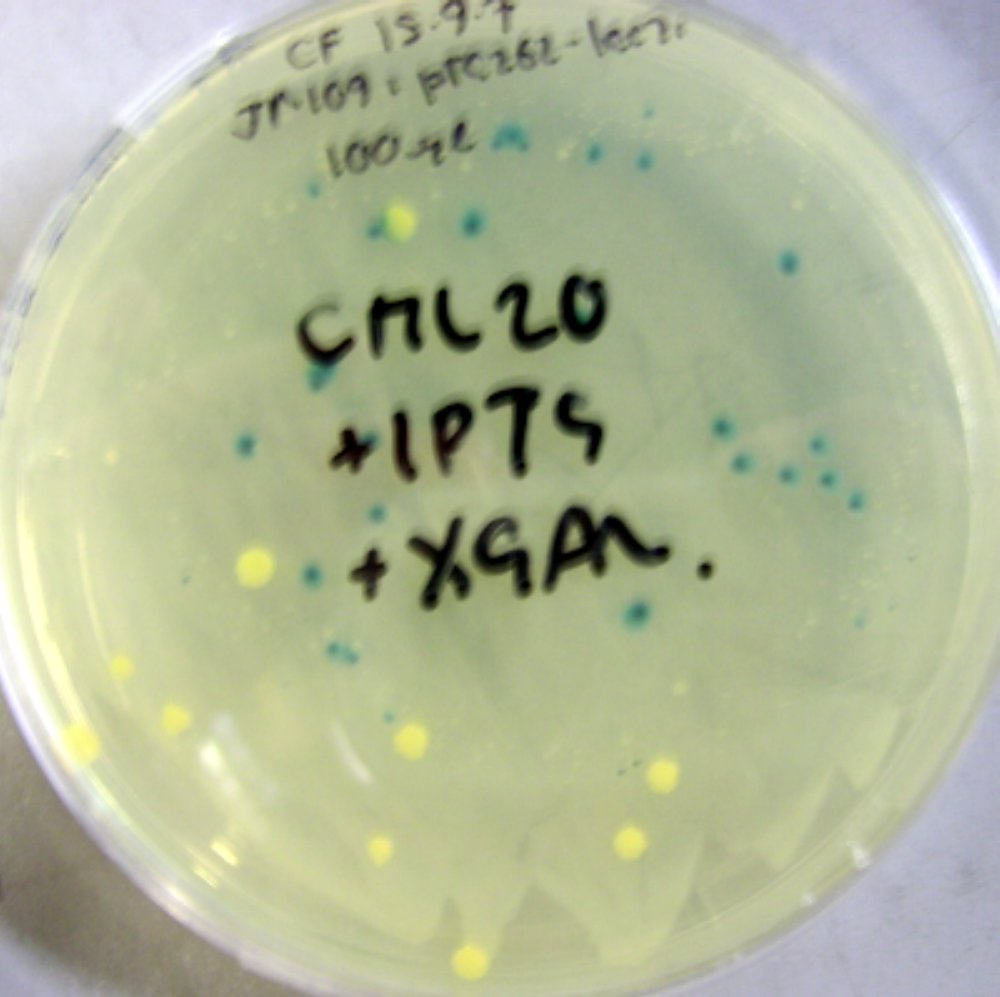
| - | + | [[Image:PTG262 Bacillus colonies.JPG|thumb|left|'''Fig. 3:''' ''Bacillus subtillis'' which has been successfully transformed with pTG262-plac-RFP vector|300 px]] [[Image:PTG262-lacz Bacillus.JPG|thumb|300 px|'''Fig. 4:''' ''E. coli'' which has been successfully transformed with lacz-pTG262 containing vector (blue colonies)]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | [[Image:PTG262 Bacillus colonies.JPG|thumb|left|'''Fig. 3:''' ''Bacillus subtillis'' which has been successfully transformed with pTG262-plac-RFP vector|300 px]] [[Image:PTG262-lacz Bacillus.JPG|thumb|300 px|'''Fig. 4:''' '' | + | |
Revision as of 12:12, 26 October 2007
 Introduction | Applications | Design | Modelling | Wet Lab | Proof of concept | References
Introduction | Applications | Design | Modelling | Wet Lab | Proof of concept | References
In order to test the feasibility of gene expression in Lactobacillus and the possibility of making self flavouring yoghurt, we required a proof of concept.
As both the pigment and flavour pathways are rather complex and will require modification and optimisation before their expression in yoghurt we planned to use the much simpler Red Fluorescent Protein (RFP) gene as a proof of concept.
So far we have managed to transform a number of RFP-pTG262 vectors containing a variety of promoters into E. coli and also Bacillus subtillis, a gram positive bacterium.
We chose to initially transform Bacillus subtillis with our proof of concept vectors over Lactobacillus for two reasons:
1. the bacterium is naturally competent at certain stages of its life cycle and therefore has a much simpler transformation process
2. members of the French lab have had successful results from when they previously worked with Bacillus subtilis
pTG262-PLac-RFP construct
We have inserted the lactose induced RFP gene into the pTG262 vector
- this vector was then transformed into E. coli
- growth on IPTG-Xgal media resulted in RFP expression and production of red colonies, these can be viewed in figure 1
pTG262-Ptet-RFP construct
We ligated the Ptet induced RFP biobrick into the pTG262 vector. We then transformed the vector into E. coli.
RFP synthesis was observed.
ptet incuded RFP E. coli colonies can be viewed in figure 2.
pTG262 expression in Bacillus subtilis
After the successful expression of both the pTG262 RFP constructs in E. coli, we decided to determine if pTG262 could be stably maintained in Bacillus subtilis.
To do this we transformed both the pTG262 and Plac-RFP-pTG262 vectors into Bacillus subtilis.
Figure 3 depicts the successful transformation and expression of pTG262 in Bacillus subtilis.
This is determined by the ability of Bacillus to grow on chloramphenicol plates.
Unfortunately RFP was not expressed from the pLac promoter, as shown by the colonies being white not red. We are attributing this to the Bacillus transcription machinary (particularly RNA polymerase and its associated sigma factors) not recognising the promoter.
Introduction | Applications | Design | Modelling | Wet Lab | Proof of concept | References