From 2007.igem.org
<Return to lab book summary>
Prepared for site dirrected mutagenesis
- Diluted DNA from last round, creating 10ng/ul sample and then adding 1uL of this to 36uL milliQ water to produce required 10ng/37uL template for PCR.
| Tube | conc of miniprep ng/ul total=(100ul) | 1uL miniprep added to x uL milliQ -> 10ng/uL
|
| 31A | 117 | 10.7
|
| 31B | 133 | 12.3
|
| 32B | 96 | 8.6
|
| 33A | 114 | 10.4
|
Site dirrected Mutagenesis Round #4
- Applied the stratagene Site directed mutagenesis protocolto the following DNA and primer pairs, which when plated out on LB AMP plates producing the numbers of colonies shown. Several of these were picked and tubes marked with a character suffix as shown in table.
Site dirrected Mutagenesis round #4
| Mutation Number | Template DNA (10ng) | Sence Primer | Antisence Primer | #Colonies | Picks named
|
| 21 | 5A | GvpL-g351a | GvpL-g351a-R | 21A,21B |
|
| 22 | 6A | GvpL-g318a | GvpL-g318a-R | 3 | 22A,22B,22C
|
| 23 | 11A | GvpQ-g183a | GvpQ-g183a-R | 26 | 23A,23B
|
| 24 | 10B | GvpP-g441a | GvpP-g441a-R | 60 | 24A,24B
|
| 25 | 6A | GvpL-g696a | GvpL-g696a-R | 1 | 25A,25B (new PCR conditions)
|
| 26 | 6A | GvpL-g213a | GvpL-g213a-R | | 26A,26B (new PCR conditions)
|
| 27 | 10B | GvpQ-g150a | GvpQ-g150a-R | | 27A,27B (new PCR conditions)
|
| 28 | 10B | GvpQ-g150a | GvpQ-g150a-R | None |
|
DNA concentrations in ng/uL
| History | Mutation tube\colony: | A | B | C | D | E
|
| pNL29T1-(Comp-g318a)-(GvpL-g351a)-> | 21 | 183
|
| pNL29T1-(GvpL-g351a)-(GvpL-g318a)-> | 22 | 176
|
| pNL26P3-(GvpP-g441a)-(GvpQ-g183a)-> | 23 | 247
|
| pNL26P3-(GvpQ-g183a)-(GvpP-g441a)-> | 24 |
|
| pNL29T1-(GvpL-g351a)-(GvpL-g696a)-> | 25 |
|
| pNL29T1-(GvpL-g351a)-(GvpL-g213a)-> | 26 |
|
| pNL26P3-(GvpQ-g183a)-(GvpQ-g150a)-> | 27 |
|
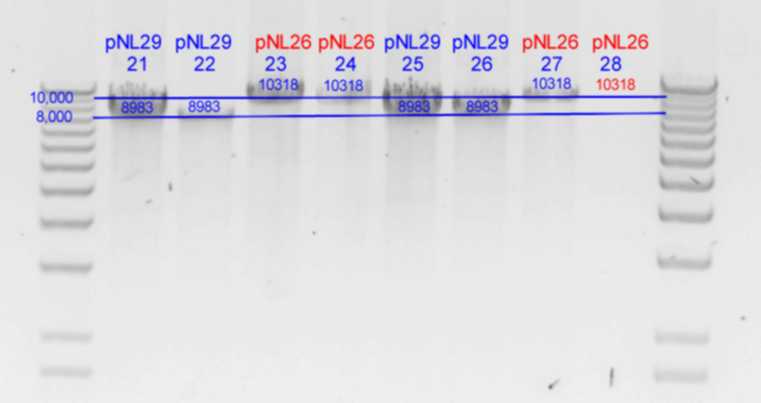
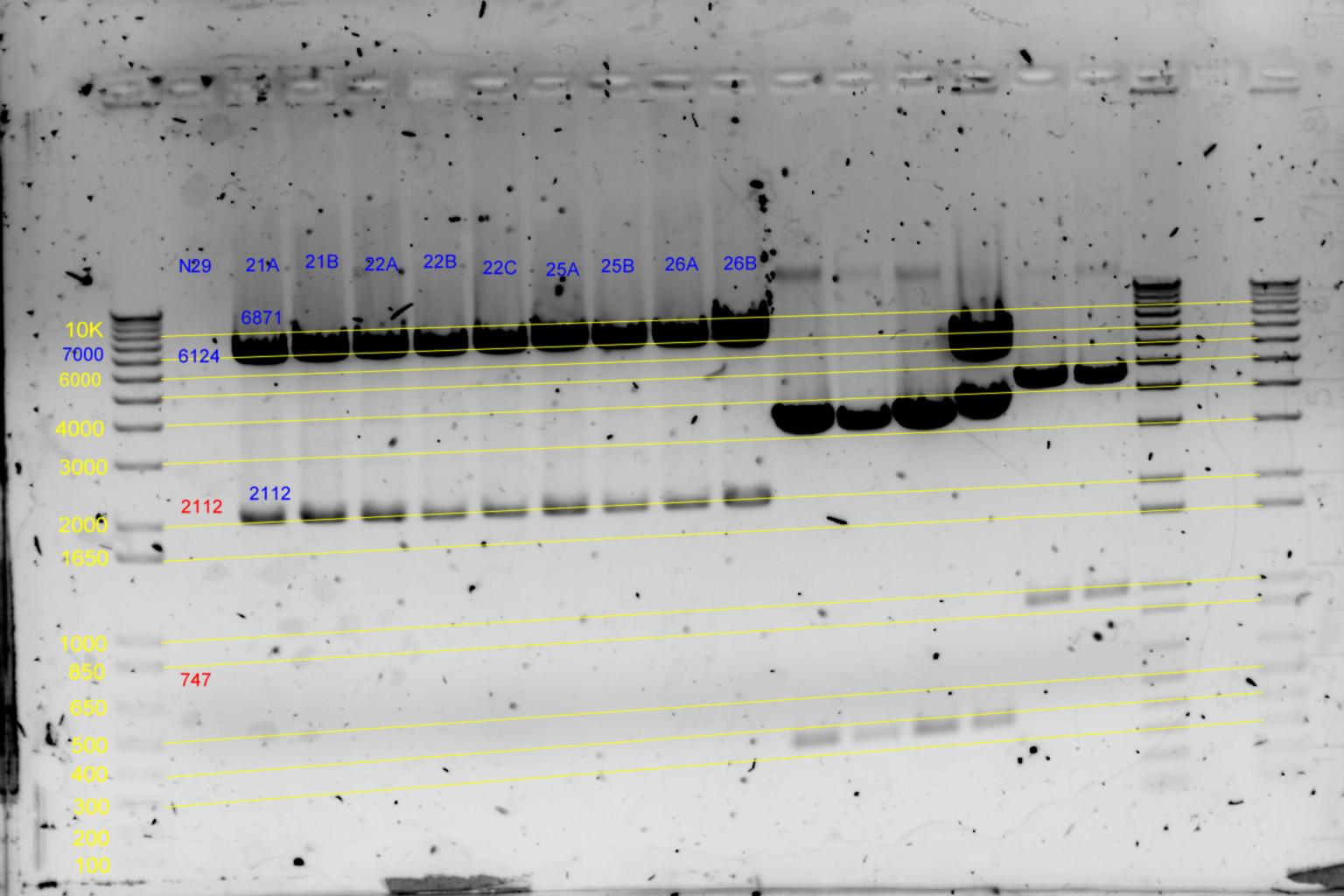
- Diagnostic digests were performed using
- A) 21.5uL of each in Buffer3 with 1uL PstI (20U) at 37degC for 2h30min. (25uL reaction)
- B) 21.5uL of each p29T1 based colony in Buffer2 with 1uL EcoRI (20U) and 1uL BamHI (20U) at 37degC for 2h30min. (26uL reaction)
- C) 21.5uL of each pNL26P3 based colony and in Buffer2 with 1uL EcoRI (20U) at 37degC for 2h30min. (25uL reaction)
- Prepared two 20 lane 100mL agarose gel
- Loaded 20uL of digest
- Digest Pattern
- Expected effects
PstI digest
| History | Mutation tube | FRAGMENTS
|
| pNL29T1-(Comp-g318a)-(GvpL-g351a)-> | 21 | | 5983 | 2516 | 483 | <-
|
| pNL29T1-(GvpL-g351a)-(GvpL-g318a)-> | 22 | | 5983 | 2516 | 483 | <-
|
| pNL26P3-(GvpP-g441a)-(GvpQ-g183a)-> | 23 | | 4148 | 3170 | 2516 | 378 | <- | 105
|
| pNL26P3-(GvpQ-g183a)-(GvpP-g441a)-> | 24 | | 4148 | 3170 | 2516 | 378 | <- | 105
|
| pNL29T1-(GvpL-g351a)-(GvpL-g696a)-> | 25 | | 5983 | 2894 | <- | 105
|
| pNL29T1-(GvpL-g351a)-(GvpL-g213a)-> | 26 | | 6088 | 2516 | 378 | <-
|
| pNL26P3-(GvpQ-g183a)-(GvpQ-g150a)-> | 27 | | 3928 | 3390 | 2516 | 378 | <- | 105
|
- Results of PstI diagnostics:
EcoRI and BamHI digest
| History | Mutation tube | Fragments:
|
| pNL29T1-(Comp-g318a)-(GvpL-g351a)-> | 21 | | 6871 | 2112 | <-
|
| pNL29T1-(GvpL-g351a)-(GvpL-g318a)-> | 22 | | 6871 | 2112 | <-
|
| pNL29T1-(GvpL-g351a)-(GvpL-g696a)-> | 25 | | 6871 | 2112 | <-
|
| pNL29T1-(GvpL-g351a)-(GvpL-g213a)-> | 26 | | 6871 | 2112 | <-
|
- Results of EcoRI diagnostics:
Conclude 21A,22A,23A are all satisfactory.