Imperial/Cell by Date/Specification
From 2007.igem.org

Cell by Date: Specifications
Cell by Date in the Cold Chain:
--Anthony L 20:12, 25 October 2007 (EDT)Would like someone to come along and make me an image of same size 900x140 pixels describing in a cool fun way the cold chain and where cell by date fits in -> Mr.Lucas Mr . Jimmi
| * Input: Isothermal & Dynamic conditions eg. steps & ramps between 0 & 40 °C Temperature is considered to be the major factor in beef spoilage and although industry tries to keep temperature low during transportaion exposure of the beef to 10 degrees celcius are not unusual. It is therefore important that our that we look at the performance of our system in isothermal conditions eg. in the cold chain, and dynamic temperature scenarios eg. a break in the cold chain.
System should give a visual signal to indicate level of thermal exposure where beef is off, to determine when beef is off we have to consider the biology of the spoilage process.The dominant organisms leading to the spoilage of beef depend of the beef's composition and the environmental conditions under which the beef is stored. For refrigerated packaged beef Pseudomonas spp. were dominate areobically while Lactobacillus was dominant anaerobically.1 Because bacteria are responsible for the spoilage of beef it is unwise to use a bacteria based device eg. by using e.coli/yeast as a chassis as this would further add to the spoilage. There also seems to be a general rule for beef that when the bacterial count reaches 107 cm-2, off odours and slime production occur and the beef is considered off. 2 For controlled isothermal conditions in a laboratory environment the time taken for beef to reach this spoilage point seems to be at most 7 days.3 This implies that the shelf life of our system needs to be at least 7 days.
The Gompertz's model is widely used when considering beef spoilage as it has been shown to fit growth data very well. 1 Using the Gompertz model we can get the specific growth rate, Lag phase duration (LPD) and maximum population density (MPD) for bacterial growth at a particular constant temperature. And then using these we can determine the Activation Energy (Ea) for the beef spoilage reaction. For U2 grade Argentinian beef stored in polyethylene and SARAN PVC, the Ea ranged from 80kJ mol-1 to 220kJ mol-1 for a range of bacteria. 4 One contrary value for the Ea of the beef spoilage rxns is given by Leak 2 who calculated Ea = 30kJ mol-1. The difference between Leak's value and that of Giannuzzi 4 probably lies in their packaging methods, which is of importance as the project aims to target aerobically fresh gound beef.
Several Technologies have already been developed to address the problem of monitoring the thermal exposure of products in the cold chain. One particular family of these products are called Temperature Time Integrators (TTI).5 The key aspect of a TTI is that they are based on a phenomenon which can act as a signal to a consumer for example, eg. a colour change. The rate at which this change occours needs to be temperature dependant so it can mimic the effect temperature has on the spoiling of meat eg. change happens quicker at higher temperatures. In order for a TTI to accurately report the spoilage rxn of beef, the activation energy of the two rxns needs to be similar. For example a difference between the two Ea's less than 20kJ mol-1 would result in the TTI estimating the thermal history of the beef to be within 1 degree C of the actual history.6
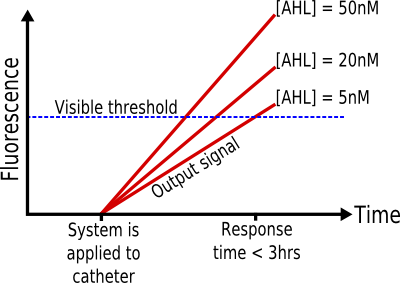
In addition, to correctly couple the Ea of our system to that of the dominant spoilage reaction in beef, we also have to consider the response time of our sytem. Our system needs to have a rapid response time, so in other words it needs to be able to quickly switch between states eg. low output and high output. This is so our system can capture small variations of temperature in the cold chain and report them in a meangingful way. Specifically, a response time in the order of a few hours would ensure that if there are any problems in the cold chain this will arise in our system very quickly.
| ||
| d | e | f |
| g | h | i |
References
- [http://www.springerlink.com/index/G311226U2M51T844.pdf Labuza TP, Fu B. Growth kinetics for shelf-life prediction: theory and practice. J of Industrial Microbiology 1993;12:309-23.]
- [http://66.102.1.104/scholar?hl=en&lr=&q=cache:thi6BTIW1YMJ:www.vitsab.com/PDF/V507.pdf+TTI+Beef+Activation+Energy Leak, F.W. (2000): Quality changes in Ground beef during distribution and storage, and determination of Time- Temperature-Indicator (TTI) charakteristic of ground beef University of Florida Institute of food and Agricultural Sciences Internet: www.vitsab.com, Stand: April 2003]
- [http://iufost.edpsciences.org/index.php?option=article&access=standard&Itemid=129&url=/articles/iufost/pdf/2006/01/iufost06000765.pdf Koutsoumanis K, Stamatiou A, Skandamis P, Nychas GJ. Development of a Microbial Model for the Combined Effect of Temperature and pH on Spoilage of Ground Meat, and Validation of the Model under Dynamic Temperature Conditions. Appl Environ Microbiol. 2006 Jan;72(1):124-34.]
- [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T7K-3S3M25D-B&_user=217827&_coverDate=01%2F06%2F1998&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=80630ba21fbc6869b9f5179d334734da Giannuzzi L, Pinotti A, Zaritzky N. Mathematical modelling of microbial growth in packaged refrigerated beef stored at different temperatures. Int J Food Microbiol. 1998 Jan 6;39(1-2):101-10.]
- [http://www.iaph.uni-bonn.de/Coldchain/downloads/06_Labuza.pdf Labuza 2006 : Cold Chain-Management II Time-temperature Integrators and the Cold chain: What is next? Bonn Germany 5/8/06]
- [http://www.blackwell-synergy.com/doi/pdf/10.1111/j.1365-2621.2001.tb15211.x?cookieSet=1 E. Shimoni, E.M. Anderson, T.P. Labuza (2001) Reliability of Time Temperature Indicators Under Temperature Abuse. Journal of Food Science 66 (9), 1337–1340. doi:10.1111/j.1365-2621.2001.tb15211.x ]
- [http://linkinghub.elsevier.com/retrieve/pii/S0168-1605(05)00067-X Giannakourou MC, Koutsoumanis K, Nychas GJ, Taoukis PS. Field evaluation of the application of time temperature integrators for monitoring fish quality in the chill chain. Int J Food Microbiol. 2005 Jul 25;102(3):323-36.]