Imperial/Cell by Date/Testing
From 2007.igem.org
(Difference between revisions)
m (→<font color=blue>Investigating properties of system under isothermal scenarios</font>) |
m (→<font color=blue>Investigating properties of system under isothermal scenarios</font>) |
||
| Line 45: | Line 45: | ||
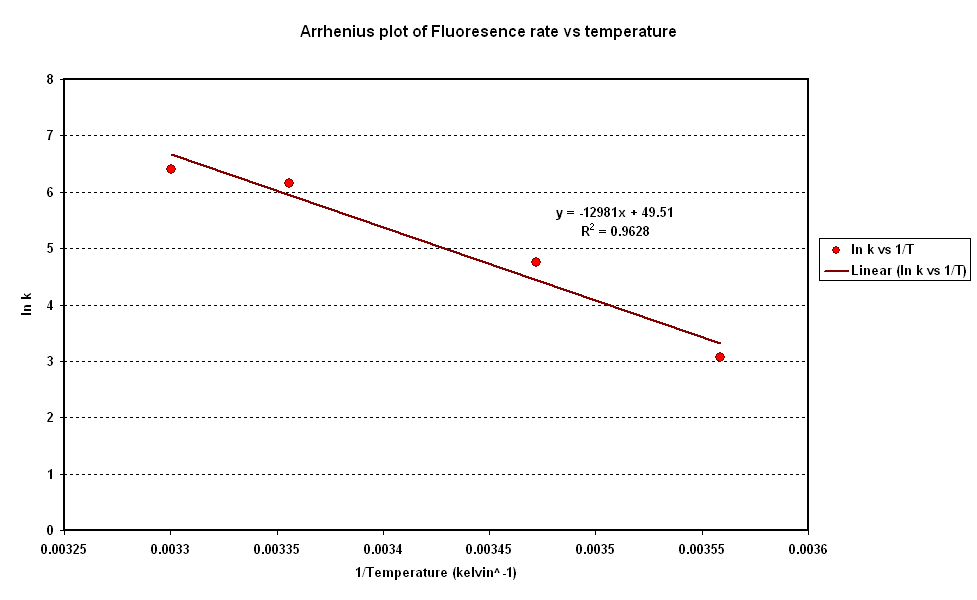
[[Image:CBD Arrhenius Plot.PNG|centre|frame|600px|Fig 2: Arrhenius Plot]] <br clear = "all" > | [[Image:CBD Arrhenius Plot.PNG|centre|frame|600px|Fig 2: Arrhenius Plot]] <br clear = "all" > | ||
| + | |||
| + | ===<font color=blue>Investigating properties of system under isothermal scenarios</font>=== | ||
| + | |||
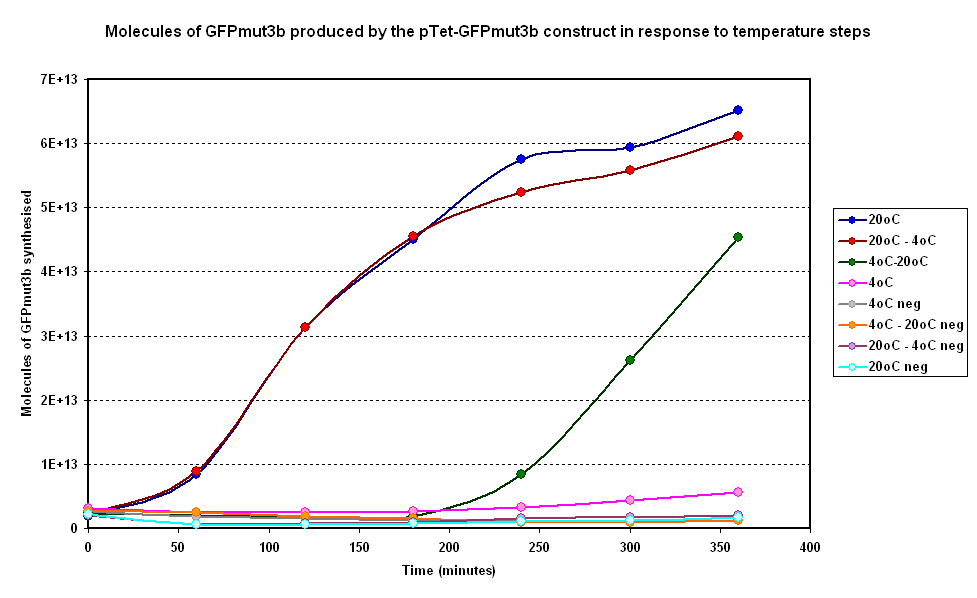
| + | [[Image:IC 2007 CBD steps.PNG|600px|center|thumb|Fig 1.1:Average fluorescence over time for two samples at constant temperatures of 4°C and 20°C and two with a temperature step (4-20°C and 20-4°C)]] | ||
'''Other practical considerations:''' <br> | '''Other practical considerations:''' <br> | ||
Revision as of 21:38, 26 October 2007

Cell by Date: Testing
Summary
Aims
Results
Investigating properties of system under isothermal scenarios
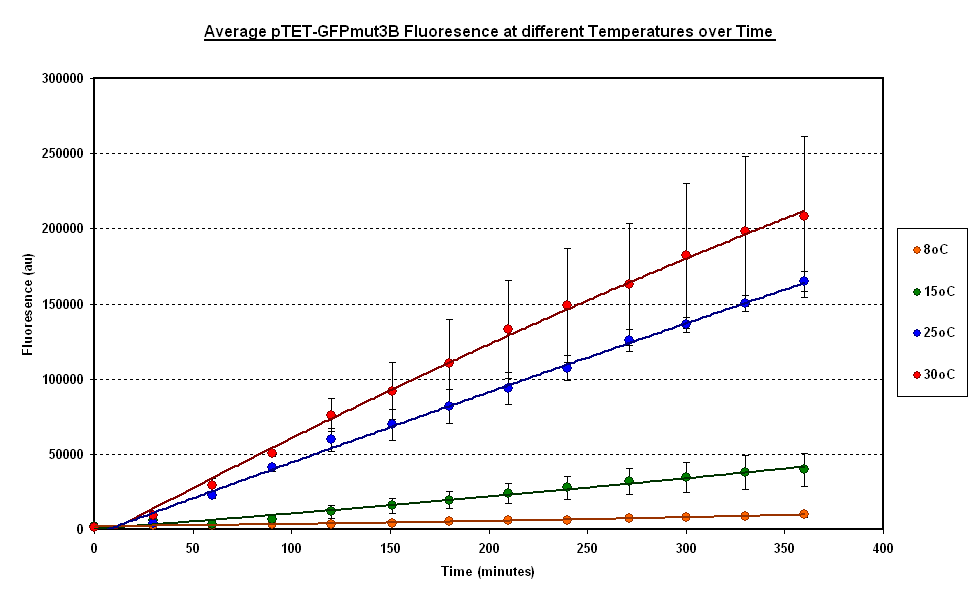
To estimate the properties of our system we looked at the evolution of the Fluorescence with time for experiments where temperature is carefully kept constant. (Figure 1).
General Behaviour:
- In all experiment we can observe a linear growth of fluorescence corresponding to a steady rate of protein production.
- This matches the claims of the manufacturer of the cell-free extract that the extract is optimised so that protein degradation is negligible.
Operating Range:
- Our system seems to 'turn off' at around 4 degrees C.
- At 8 degrees with have minimal expression which then increases to a substantial expression at 37 degrees.
Synthesis rate vs Temperature
- Over the range of temperature considered here the rate increases with temperature.
- To investigate the relation between synthesis rate and temperature we extracted the rate of synthesis of GFP for each experiment and plotted it against 1/T (Figure 2).
- A strong linear correlation in the log plot supports an arrhenius type dependence on temperature with an activation energy of 1.5kJ/mol.
Investigating properties of system under isothermal scenarios
Other practical considerations:
- Unfortunetly we have not been able to use a visible reporter and so have no idea whether the construct can in fact produce a visible signal.
- In terms of lifespan we have had major problems with evaporation meaning that the lifespan of our system is limited to a few days.