Imperial/Infector Detector/Testing
From 2007.igem.org
(Difference between revisions)
m (→DNA Concentrations) |
m (→DNA Concentrations) |
||
| Line 35: | Line 35: | ||
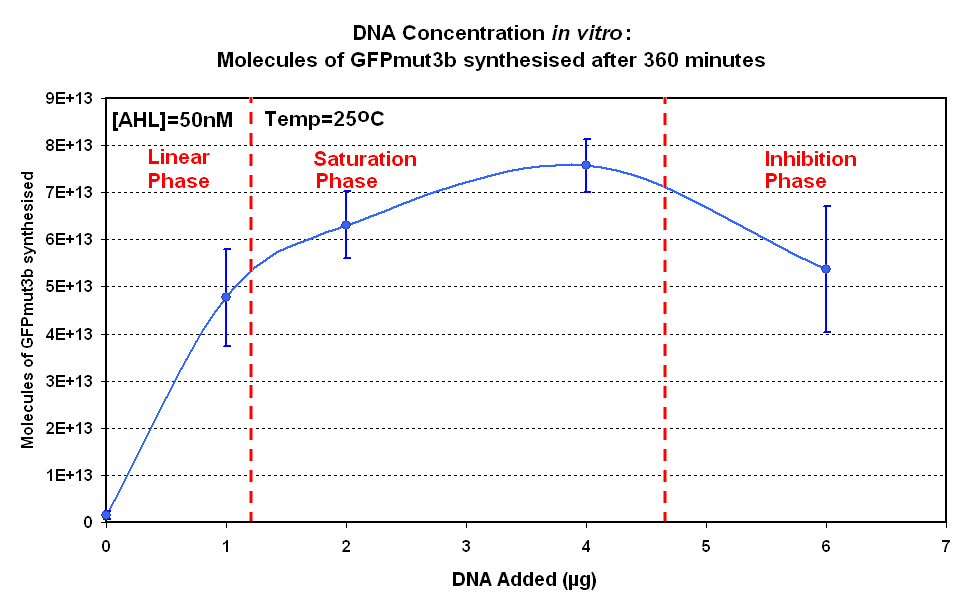
| width="50%"|<br>[[image:IC 2007 DNA Concentration 360mins.PNG|thumb|450px|Fig.1.2:Molecules of GFPmut3b synthesised for each DNA Concentration ''in vitro'', after 360 minutes.]] | | width="50%"|<br>[[image:IC 2007 DNA Concentration 360mins.PNG|thumb|450px|Fig.1.2:Molecules of GFPmut3b synthesised for each DNA Concentration ''in vitro'', after 360 minutes.]] | ||
|} | |} | ||
| + | |||
| + | The Results above show that the optimum DNA concentration for ''in vitro'' is 4µg | ||
===Testing AHL Range=== | ===Testing AHL Range=== | ||
Revision as of 13:32, 26 October 2007

Infector Detector: Testing
Aims
The aims of the testing were as follows:
- To test and obtain the optimal DNA concentration for construct 1 in vitro
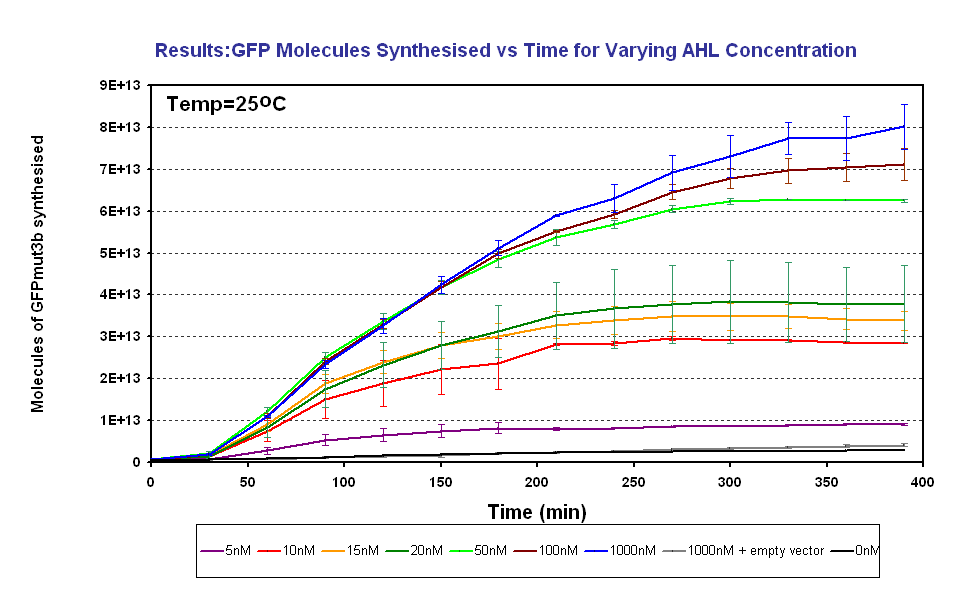
- To characterise the output of GFPmut3b for a range of AHL inputs. From this obtain the AHL sensitivity of our system.
In addition the fluorescence measurements were converted to number of GFPmut3b molecules synthesised using a calibration curve constructed using purified GFPmut3b.
Results
DNA Concentrations
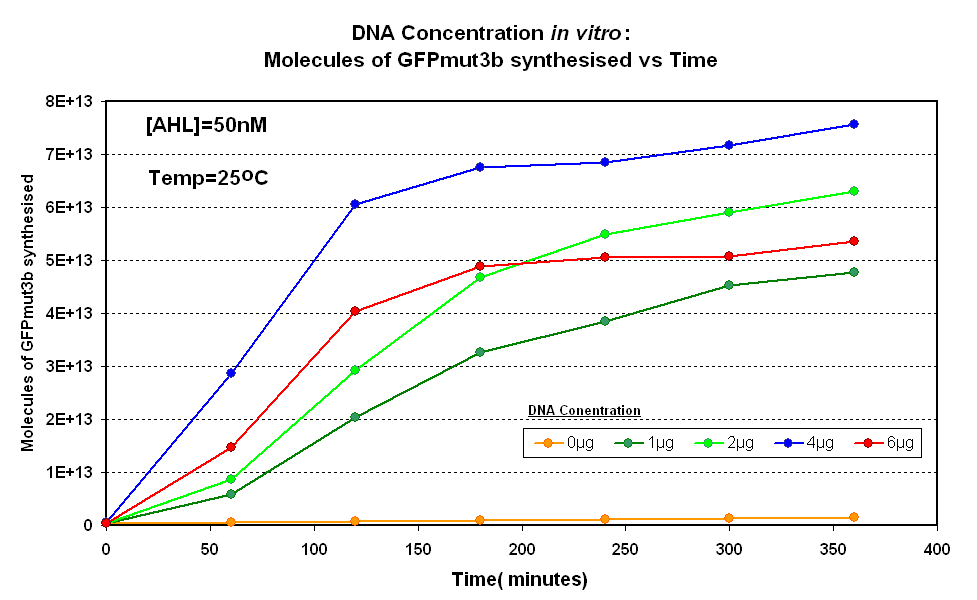 Fig.1.1:Molecules of GFPmut3b synthesised over time, for each DNA Concentration in vitro - The fluorescence was measured over time for each experiment and converted into molecules of GFPmut3b in vitro using our calibration curve. |
The Results above show that the optimum DNA concentration for in vitro is 4µg
Testing AHL Range
The results show us the following:
|