Imperial/Wet Lab/Protocols/CBD1.1
From 2007.igem.org
(Difference between revisions)
m (→Protocol) |
m |
||
| Line 4: | Line 4: | ||
= Wet Lab: Protocols: Initial Testing of DNA Constructs ''in vivo'' = | = Wet Lab: Protocols: Initial Testing of DNA Constructs ''in vivo'' = | ||
| - | + | ==Aims== | |
| - | + | ||
*To test to see if the DNA constructs from the registry are viable. This is done in vivo. | *To test to see if the DNA constructs from the registry are viable. This is done in vivo. | ||
*The constructs are pTet-GFP and pT7-GFP. | *The constructs are pTet-GFP and pT7-GFP. | ||
| - | + | ||
| - | + | ==Day 1== | |
| + | ===Equipment=== | ||
*7ml sterile tubes x4 | *7ml sterile tubes x4 | ||
*1.5ml Eppendorf tube x1 | *1.5ml Eppendorf tube x1 | ||
| Line 15: | Line 15: | ||
*Gilson Pipettes p1000 p200 p20 | *Gilson Pipettes p1000 p200 p20 | ||
| - | + | ===Reagents=== | |
*''E.coli'' BL21; culture containing parts :pTet-GFP, pT7-GFP, pcI-GFP | *''E.coli'' BL21; culture containing parts :pTet-GFP, pT7-GFP, pcI-GFP | ||
*LB medium | *LB medium | ||
| Line 23: | Line 23: | ||
<br> | <br> | ||
| - | + | ===Protocol=== | |
'''Innoculation of Media''' | '''Innoculation of Media''' | ||
#Inoculate 10ul of transformed E.coli cells in individual 2ml LB medium containing 2ul of ampicillin | #Inoculate 10ul of transformed E.coli cells in individual 2ml LB medium containing 2ul of ampicillin | ||
| Line 29: | Line 29: | ||
<br> | <br> | ||
| - | + | ==Day 2== | |
| - | + | ===Equipment=== | |
*1 Fluorometer plate (black) | *1 Fluorometer plate (black) | ||
*Fluorometer + PC | *Fluorometer + PC | ||
| Line 36: | Line 36: | ||
*Eppendorf tubes | *Eppendorf tubes | ||
| - | + | ===Reagents=== | |
*LB medium | *LB medium | ||
*E.coli culture with transformed plasmid | *E.coli culture with transformed plasmid | ||
| Line 43: | Line 43: | ||
<br> | <br> | ||
| - | + | ===Protocol=== | |
'''Preparation of diluted GFP standard solution'''<br> | '''Preparation of diluted GFP standard solution'''<br> | ||
#Add 995ul of ultra pure water an eppendorf tube, together with 5ul of undiluted GFP standard solution and mix. '''(This gives a 200x dilution to be used as a positive control)''' | #Add 995ul of ultra pure water an eppendorf tube, together with 5ul of undiluted GFP standard solution and mix. '''(This gives a 200x dilution to be used as a positive control)''' | ||
| Line 50: | Line 50: | ||
<br> | <br> | ||
| - | + | ====Loading Plate==== | |
| - | + | ||
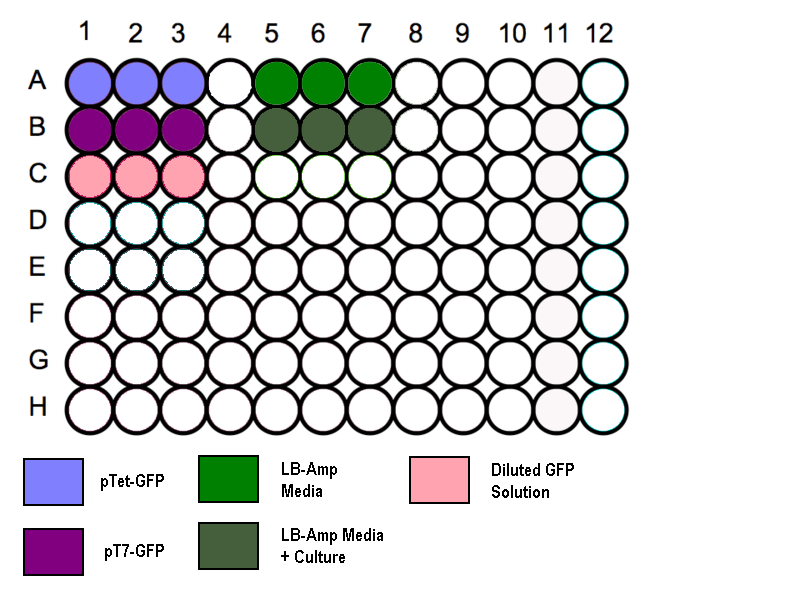
#Transfer 200 µl aliquots of each of the cultures to a flat-bottomed 96 well plate. '''(Follow the schematic as shown)''' | #Transfer 200 µl aliquots of each of the cultures to a flat-bottomed 96 well plate. '''(Follow the schematic as shown)''' | ||
#Three wells to be filled with 200µl of media to measure the absorbance background. | #Three wells to be filled with 200µl of media to measure the absorbance background. | ||
Revision as of 20:23, 26 October 2007

Wet Lab: Protocols: Initial Testing of DNA Constructs in vivo
Aims
- To test to see if the DNA constructs from the registry are viable. This is done in vivo.
- The constructs are pTet-GFP and pT7-GFP.
Day 1
Equipment
- 7ml sterile tubes x4
- 1.5ml Eppendorf tube x1
- 37°C incubator
- Gilson Pipettes p1000 p200 p20
Reagents
- E.coli BL21; culture containing parts :pTet-GFP, pT7-GFP, pcI-GFP
- LB medium
- Ampicillin stock (50 mg/ml)
Protocol
Innoculation of Media
- Inoculate 10ul of transformed E.coli cells in individual 2ml LB medium containing 2ul of ampicillin
- Incubate at 37°C for overnight in a shaker. (This is to get a good stock of cells for use in the experiment. After the overnight culture the cells will be in stationary phase)
Day 2
Equipment
- 1 Fluorometer plate (black)
- Fluorometer + PC
- Gilson pipettes 1000 and 200
- Eppendorf tubes
Reagents
- LB medium
- E.coli culture with transformed plasmid
- GFP standard solution
- ddH2O
Protocol
Preparation of diluted GFP standard solution
- Add 995ul of ultra pure water an eppendorf tube, together with 5ul of undiluted GFP standard solution and mix. (This gives a 200x dilution to be used as a positive control)
- Place the tube on ice till it is ready to be used.
Loading Plate
- Transfer 200 µl aliquots of each of the cultures to a flat-bottomed 96 well plate. (Follow the schematic as shown)
- Three wells to be filled with 200µl of media to measure the absorbance background.
- Standard GFP solution added as a positive control.
- Remove lid and measure in the flourometer.
- (Fluorescence measurements - 488 nm excitation filter, 525 nm emission filter, 0.5 seconds, CW lamp energy 12901 units)
- Repeat the measurement a further two times straight after each other (This is to test the variability of the machine)
Schematic
|