Imperial/Wet Lab/Protocols/CBD2.1
From 2007.igem.org
(Difference between revisions)
| Line 1: | Line 1: | ||
| + | {{Template: IC07navmenu}} | ||
| + | __NOTOC__ | ||
| + | |||
| + | = Wet Lab: Protocols: Initial Testing for Operating Temperature Range = | ||
| + | |||
| + | |||
'''Aims''' | '''Aims''' | ||
*To determine if construct expresses ''in vitro'' at temperatures of: 10<sup>o</sup>C, 20<sup>o</sup>C, 37<sup>o</sup>C | *To determine if construct expresses ''in vitro'' at temperatures of: 10<sup>o</sup>C, 20<sup>o</sup>C, 37<sup>o</sup>C | ||
Revision as of 04:29, 22 October 2007

Wet Lab: Protocols: Initial Testing for Operating Temperature Range
Aims
- To determine if construct expresses in vitro at temperatures of: 10oC, 20oC, 37oC
- To determine specific life span at each temperature range.
- To determine the maximum rate of GFP produced at each temperature range.
Equipments
- Fluorometer + PC
- Water bath in cold room at 10°C/20°C
- 37°C incubator
- 3 Fluorometer plates (black)
- Gilson pipettes p200 p20 p10
- Eppendorf Tubes
- Stopwatch
Reagents
- Commercial S30 E.coli extract. Including:
- 175µl Amino Acid Mixture Minus Methionine, 1mM
- 175µl Amino Acid Mixture Minus Leucine, 1mM
- 450µl S30 Extract, Circular (3 × 150µl)
- 750µl S30 Premix Without Amino Acids
- DNA pTet-GFP from midiprep
- Nuclease Free water
- GFP solution
Protocols
- First collect all equipment and reagents and ensure that the fluorometer and that the PC connected has a data collection protocol installed.
- Place each of the 96 well plates together with their plate mates in their respective incubators so as to heat them up to the appropriate temperature before the experiments start.
- For the next step of the go to the biochemistry level 5 and remove:
- A.A's from kits
- 2xPremix tubes (60ul each)
- 2xS30 tubes (45ul each)
- For Each Temperature Carry out the following Procedure
- Commercial E.coli Cell Extract: First prepare a complete amino acid mixture for both extract solutions: Add the 10μl volume of two amino acid minus mixtures into an labeled eppendorf to give a volume of 20μl. Each amino acid minus mixture is missing one type of amino acid, and so by combining two solutions we are complementing each solution for the missing amino acid. Place eppendorf in a rack on bench.
- Commercial E.coli Cell Extract:Take an eppendorf tube and add 20µl of the E.coli complete amino acid mixture. Then add 80µl of S30 Premix Without Amino Acid. Then add 60µl of S30 Extract Circular. Place the eppendorf tube in a rack on the bench.
- Vortex the tubes to mix thoroughly and place the tubes of E.coli commercial extract in each incubator for ten minutes.
- Separate 80μl of midipreped DNA plasmid into an eppendorf tube and place them in their respective incubators as well.
- Separate 20ul of midipreped DNA of empty vector into separate tubes.
- Any left over premix or cell extract should be returned to the freezer in biochemistry level 5 and labeled with new volumes.
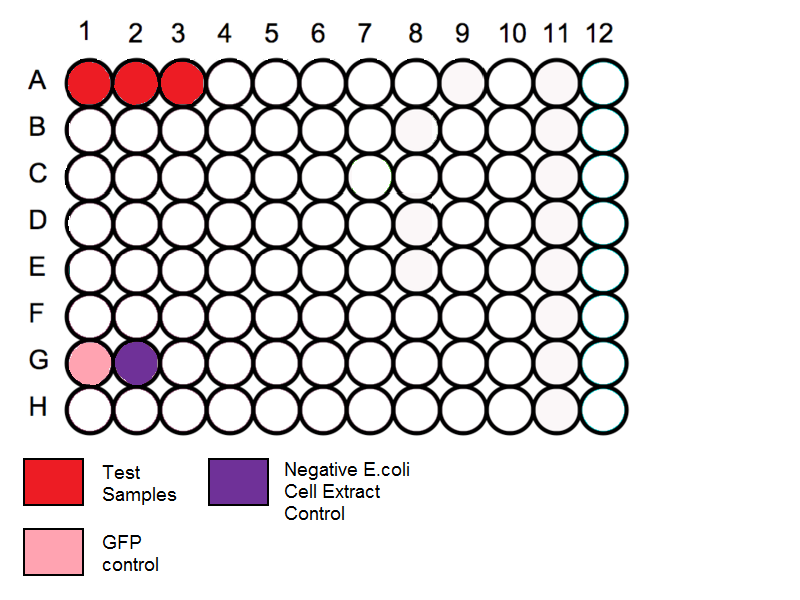
Loading Plate
- Follow the schematic for the plate and begin by loading the in vitro expression system into the correct wells. Before loading in the samples vortex the tubes for a few seconds to mix the solution.
- Tap down the top of the lid to bring down any solution to bottom of the well.
- Remove lid off the 96 well plate and place in the fluorometer. Create a file name insert temp under: D:\IGEM\INSERT DATE\ID. Export the data here. Each file should be named as the following:
- construct-temp-time-date
- This measurement will give a back ground fluorescence measurement and can be used as our time zero data.
- Then to begin the reaction add 20μl of purified DNA sample to each well indicated on the schematic. Be careful not to add to wells that DO NOT NEED DNA.
- Place lid back on and place back in the respective incubators.
- After 10 minutes of incubation measure the fluorescence by repeating procedure 3-4 above. This initial measurement of 10 minutes should be carried on for 1 hours or until it appears GFP production levels off.
- Before each measurement be careful to remember to tap down the solution and to remove the lid before placing in the fluorometer.
Schematic
Plan for Temperature Testing Day 1-3
- 1st Day - 20, 37
- 2nd Day - 15, 25, 30, 40
- 3rd Day - 10, 45
|