Imperial/Infector Detector/Specification
From 2007.igem.org
(→Specifications in detail) |
(→Infector Detector: Specifications) |
||
| Line 42: | Line 42: | ||
In order for the system to be used by a nurse, without needing any special equipment, the output signal must be visible. | In order for the system to be used by a nurse, without needing any special equipment, the output signal must be visible. | ||
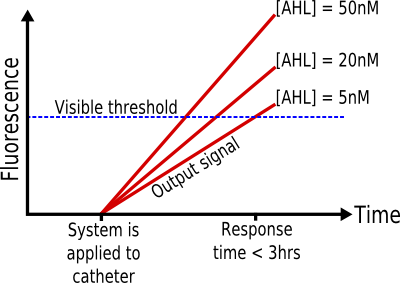
* '''Response Time: < 3 hours''' | * '''Response Time: < 3 hours''' | ||
| - | Given that biofilms grow and spread in a period of hours and days, the response time of the system must be as short as possible. The threshold of three hours is achievable in the time constraints of protein expression systems, yet it is short enough in comparison to the growth properties of biofilms. | + | Given that biofilms grow and spread in a period of hours and days, the response time of the system must be as short as possible. The threshold of three hours is achievable in the time constraints of protein expression systems, yet it is short enough in comparison to the growth properties of biofilms. ([[#References|2-3]]) |
* '''Operating conditions: Temperatures from 20° to 30°C''' | * '''Operating conditions: Temperatures from 20° to 30°C''' | ||
* '''Health & safety: Non-living, non-infectious''' | * '''Health & safety: Non-living, non-infectious''' | ||
| Line 52: | Line 52: | ||
# Charlton, TS, et al. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography–mass spectrometry: application to a model bacterial biofilm. Environmental Microbiology 2 (5), 530–541. October 2000. | # Charlton, TS, et al. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography–mass spectrometry: application to a model bacterial biofilm. Environmental Microbiology 2 (5), 530–541. October 2000. | ||
| + | # Stickler, DJ, et al. A Sensor To Detect the Early Stages in the Development of Crystalline Proteus mirabilis Biofilm on Indwelling Bladder Catheters. Journal of Clinical Microbiology, April 2006, p. 1540-1542, Vol. 44, No. 4. | ||
| + | # [http://inls.ucsd.edu/~volfson/biofilm/ Growth and ordering of biofilms in controlled environments] available online on 22.10.2007 | ||
Revision as of 22:18, 22 October 2007

Infector Detector: Specifications
The system must be able to detect the presence of biofilms on urinary catheters by detection of AHL, at a minimum concentration of 5nM, and report with a visual signal within 3 hours. It must work within a temperature range of 20°-30°C, be portable and easy to use, have a shelf life of at least seven days, and must not be harmful or infectious.
| Inputs | |
| Outputs | |
| Response Time | |
| Operating Conditions | |
| Health & Safety | |
| Lifespan | |
| Packaging |
Specifications in detail
- Input: AHL 5-50nM
It is known that, in Pseudomonas aeruginosa biofilms, the concentration of AHL is at least 5nM. (1) Therefore, if the system can successfully report the presence of this concentration, it should be able to detect such biofilms.
- Output: Visible fluorescent protein
In order for the system to be used by a nurse, without needing any special equipment, the output signal must be visible.
- Response Time: < 3 hours
Given that biofilms grow and spread in a period of hours and days, the response time of the system must be as short as possible. The threshold of three hours is achievable in the time constraints of protein expression systems, yet it is short enough in comparison to the growth properties of biofilms. (2-3)
- Operating conditions: Temperatures from 20° to 30°C
- Health & safety: Non-living, non-infectious
- Packaging and shelf-life of 7 days
References
- Charlton, TS, et al. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography–mass spectrometry: application to a model bacterial biofilm. Environmental Microbiology 2 (5), 530–541. October 2000.
- Stickler, DJ, et al. A Sensor To Detect the Early Stages in the Development of Crystalline Proteus mirabilis Biofilm on Indwelling Bladder Catheters. Journal of Clinical Microbiology, April 2006, p. 1540-1542, Vol. 44, No. 4.
- [http://inls.ucsd.edu/~volfson/biofilm/ Growth and ordering of biofilms in controlled environments] available online on 22.10.2007