Imperial/Infector Detector/Conclusion
From 2007.igem.org
(→Added control - Construct 2) |
|||
| (69 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Template:IC07navmenu}} | {{Template:IC07navmenu}} | ||
| + | <br clear="all"> | ||
| + | <html> | ||
| + | <link rel="stylesheet" href="/igem07/index.php?title=User:Dirkvs/Stylesheets/IC07persist.css&action=raw&ctype=text/css" type="text/css" /> | ||
| + | <div id="tabs"> | ||
| + | <ul> | ||
| + | <li><a href="https://2007.igem.org/Imperial/Infector_Detector/Introduction" title=""><span>Introduction</span></a></li> | ||
| + | <li><a href="https://2007.igem.org/Imperial/Infector_Detector/Specification" title=""><span>Specifications</span></a></li> | ||
| + | <li><a href="https://2007.igem.org/Imperial/Infector_Detector/Design" title=""><span>Design</span></a></li> | ||
| + | <li><a href="https://2007.igem.org/Imperial/Infector_Detector/Modelling" title=""><span>Modelling</span></a></li> | ||
| + | <li><a href="https://2007.igem.org/Imperial/Infector_Detector/Implementation" title=""><span>Implementation</span></a></li> | ||
| + | <li><a href="https://2007.igem.org/Imperial/Infector_Detector/Testing" title=""><span>Testing</span></a></li> | ||
| + | <li><a href="https://2007.igem.org/Imperial/Infector_Detector/F2620 Comparison" title=""><span>F2620</span></a></li> | ||
| + | <li><a class="current" href="https://2007.igem.org/Imperial/Infector_Detector/Conclusion" title=""><span>Conclusion</span></a></li> | ||
| + | </ul> | ||
| + | </div> | ||
| + | <hr /> | ||
| + | <br clear="all"> | ||
| + | </html> | ||
| + | __NOTOC__ | ||
| + | =Infector Detector: Conclusion = | ||
| + | The main achievements of the Infector Detector project: | ||
| + | *Extensive modelling of the two potential constructs for Infector Detector | ||
| + | *Purification of GFPmut3b to allow construction of a calibration curve | ||
| + | *Detailed characterisation of construct 1 ''in vitro'' using a calibration curve to find rate of GFP synthesis | ||
| + | *Creation of a standard unit to allow comparison between ''in vitro'' and ''in vivo'' | ||
| - | + | The table below summarises our Infector Detector system in the context of the original specifications: | |
| + | {|border="1" width="80%" align="center" | ||
| + | |- | ||
| + | |width="20%" style="background:#ffffcc"|<center>'''Property'''</center> | ||
| + | |width="--"|<center>'''Specification'''</center> | ||
| + | |'''Achievements''' | ||
| + | |- | ||
| + | |style="background:#ffffcc"|Inputs | ||
| + | |<center>System must be sensitive to AHL concentration between 5-50nM</center> | ||
| + | |'''<font color=green>Sensitive to 5-1000nM</font>''' | ||
| + | |- | ||
| + | |style="background:#ffffcc"|Outputs | ||
| + | |<center>System must give a visual signal if bacteria is present</center> | ||
| + | |'''<font color=red>To Be Determined - Using Stronger fluorescent protein such as DsRed express</font>''' | ||
| + | |- | ||
| + | |style="background:#ffffcc"|Response Time | ||
| + | |<center>System needs to have a response time under 3 hours</center> | ||
| + | |'''<font color=green>Systems responds <30minutes and reaches peak fluorescence at 300minutes'''</font> | ||
| + | |- | ||
| + | |style="background:#ffffcc"|Operating Conditions | ||
| + | |<center>System must operate within temperature 20-30°C</center> | ||
| + | |'''<font color=green>System works at 25°C</font>''' | ||
| + | |- | ||
| + | |style="background:#ffffcc"|Health & Safety | ||
| + | |<center>System Must not be living, replicating bacteria, and in any way harmful or infectious.</center> | ||
| + | |'''<font color=green>Cell Free ''in vitro'' chassis</font>''' | ||
| + | |- | ||
| + | |style="background:#ffffcc"|Shelf-life | ||
| + | |<center>System must have a shelf life of 7 days</center> | ||
| + | |'''<font color=green>Can be stored in freezer for prolonged periods</font>''' | ||
| + | |- | ||
| + | |style="background:#ffffcc"|Packaging and Application | ||
| + | |<center>System must be portable and convenient to use</center> | ||
| + | |'''<font color=red>To Be Determined- Using our chassis in a spray</font>''' | ||
| + | |} | ||
| + | <br clear="all"> | ||
| + | Infector detector utilises synthetic biology to fight one of the most common infections rampaging through hospitals worldwide. Urinary catheter infections and catheter-related bacteremias in general have been troubling both doctors and patients for years now on since the infection is usually very difficult to detect at an early stage. Infector detector battles the infection with its own weapons by utilising its quorum sensing mechanism to initiate a fluorescence reporter, signaling the presence of a biofilm in the making. Upon noticing the change in colour, medical staff will replace the catheter before the infection spreads. Thus doctors can rest assured that their patients get the protection they need. And all this without invoking a single bacterium, thanks to our Cell-free chassis. | ||
| + | Its evolution cannot however stop here ! While charactrising infector detector we came across certain aspects that can be further developed to enhance its functions. These are described in more detail further below: | ||
| - | |||
| + | ==Battle a spectrum of infections== | ||
| + | [[Image:IC2007 conclusion1.jpg|520px|left|]] | ||
| + | The great potential of Infector Detector lies in that it isn't limited to just one type of infection. Adding sensitivity to AHL originating from biofilms is just the beginning. By using different homologues to the LuxR quorum sensing, Infector Detector can be used to battle a range of catheter-related bacteria. For example by using, a construct that recognises AI-2<sup>[[#References |1]]</sup> we can detect the presence of Klebsiella pneumoniae, a pathogenic bacterium ranked second to E. coli for urinary tract infections in older persons. | ||
| + | <br> | ||
| + | <br clear="all"> | ||
| + | <br><br> | ||
| + | ==Added control - Construct 2== | ||
| + | <br> | ||
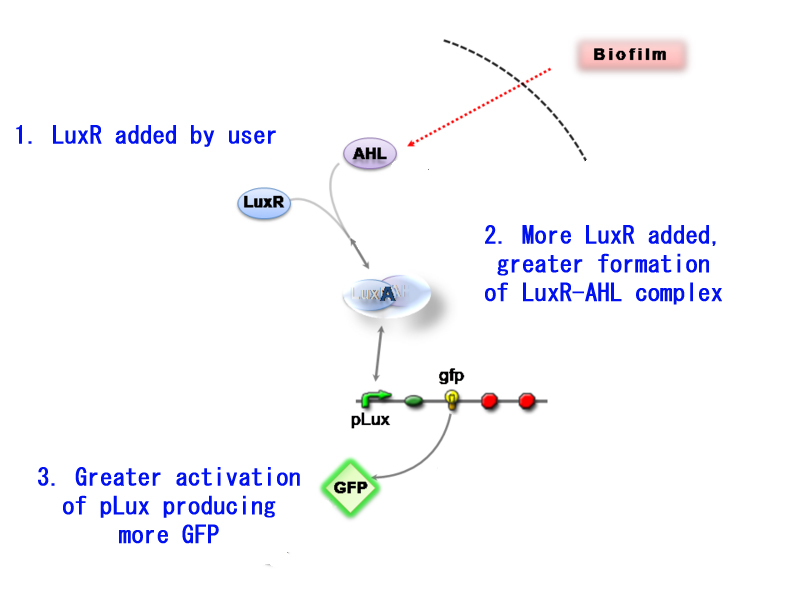
| + | [[Image:IC2007 Conculsion4.jpg|thumb|right|390px|Tweaking sensitivity using LuxR]] | ||
| + | The main advantages of using construct 2 is that it provides an additional control mechanism for our detector meaning that you can tweak the detector sensitivity. In addition by adding purified LuxR the chassis does not have to produce LuxR and so has more energy to produce GFP.<br> | ||
| + | Going into deeper detail, construct 1 can produce LuxR as soon as it is activated. LuxR's presence is necessary for the formation of AHL-LuxR complex and the subsequent activation of pLux (leading to GFP production). Construct 2 on the other hand does not have a LuxR producing part. It relies on the user to add the necessary LuxR to form the binding complex. This control over LuxR can thus act as a sort of attenuator to the sensitivity of Infector Detector. | ||
| + | Having little LuxR present, will form very little binding complex with AHL and thus the sensitivity will decrease significantly. Saturating the detection compound with LuxR will maximise the sensitivity. | ||
| + | <br clear="all"> | ||
| + | <br><br> | ||
| - | === | + | ==Packaging== |
| - | + | ||
| - | + | Infector Detector can be packaged as either a cream or a spray. | |
| + | <br>[[Image:IC2007 IDspray.jpg|thumb|150px|left|Infector Detector Spray]] | ||
| + | [[Image:IC2007_IDPackaging.jpg|thumb|150px|right|Infector Detector Creme]] | ||
| + | A spray will provide easy application of the detector because it does not require the user to fiddle around with the urinary catheter as they can simply spray from a distance. The disadvantage is the poor accuracy of application, waste, and higher rate of evaporation. | ||
| + | |||
| + | |||
| + | A cream, on the other hand, will decrease significantly any evaporation and will allow the user to apply the detector to specific areas of the catheter with more control. The disadvantage is that the diffusion rate of AHL and detection compounds through a viscous cream is lower. This will slow down system response. | ||
<br> | <br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | === | + | |
| + | |||
| + | |||
| + | Both applications provide some advantages and disadvantages that must be weighed depending on the actual use scenario of Infector Detector in order to decide which is best. | ||
| + | |||
| + | As you can see, the development of the application can easily be extended into many areas, and even though it is far from being commercially available, Infector Detector has proven that even the tiniest of members of the microcosm around us can tackle a problem of worldwide dimensions. | ||
| + | |||
| + | <br clear="all"> | ||
| + | <br clear="all"> | ||
| + | <br clear="all"> | ||
| + | <center> [https://2007.igem.org/Imperial/Infector_Detector/F2620_Comparison << F2620 Comparison] | Conclusions | [https://2007.igem.org/Imperial Home >>] | ||
| + | </center> | ||
| + | |||
| + | == References == | ||
| + | |||
| + | # Damien Balestrino et al. Characterization of Type 2 Quorum Sensing in Klebsiella pneumoniae and Relationship with Biofilm Formation. J Bacteriol. 2005 April; 187(8): 2870–2880. | ||
Latest revision as of 03:26, 27 October 2007

Infector Detector: Conclusion
The main achievements of the Infector Detector project:
- Extensive modelling of the two potential constructs for Infector Detector
- Purification of GFPmut3b to allow construction of a calibration curve
- Detailed characterisation of construct 1 in vitro using a calibration curve to find rate of GFP synthesis
- Creation of a standard unit to allow comparison between in vitro and in vivo
The table below summarises our Infector Detector system in the context of the original specifications:
| Achievements | ||
| Inputs | Sensitive to 5-1000nM | |
| Outputs | To Be Determined - Using Stronger fluorescent protein such as DsRed express | |
| Response Time | Systems responds <30minutes and reaches peak fluorescence at 300minutes | |
| Operating Conditions | System works at 25°C | |
| Health & Safety | Cell Free in vitro chassis | |
| Shelf-life | Can be stored in freezer for prolonged periods | |
| Packaging and Application | To Be Determined- Using our chassis in a spray |
Infector detector utilises synthetic biology to fight one of the most common infections rampaging through hospitals worldwide. Urinary catheter infections and catheter-related bacteremias in general have been troubling both doctors and patients for years now on since the infection is usually very difficult to detect at an early stage. Infector detector battles the infection with its own weapons by utilising its quorum sensing mechanism to initiate a fluorescence reporter, signaling the presence of a biofilm in the making. Upon noticing the change in colour, medical staff will replace the catheter before the infection spreads. Thus doctors can rest assured that their patients get the protection they need. And all this without invoking a single bacterium, thanks to our Cell-free chassis.
Its evolution cannot however stop here ! While charactrising infector detector we came across certain aspects that can be further developed to enhance its functions. These are described in more detail further below:
Battle a spectrum of infections
The great potential of Infector Detector lies in that it isn't limited to just one type of infection. Adding sensitivity to AHL originating from biofilms is just the beginning. By using different homologues to the LuxR quorum sensing, Infector Detector can be used to battle a range of catheter-related bacteria. For example by using, a construct that recognises AI-21 we can detect the presence of Klebsiella pneumoniae, a pathogenic bacterium ranked second to E. coli for urinary tract infections in older persons.
Added control - Construct 2
The main advantages of using construct 2 is that it provides an additional control mechanism for our detector meaning that you can tweak the detector sensitivity. In addition by adding purified LuxR the chassis does not have to produce LuxR and so has more energy to produce GFP.
Going into deeper detail, construct 1 can produce LuxR as soon as it is activated. LuxR's presence is necessary for the formation of AHL-LuxR complex and the subsequent activation of pLux (leading to GFP production). Construct 2 on the other hand does not have a LuxR producing part. It relies on the user to add the necessary LuxR to form the binding complex. This control over LuxR can thus act as a sort of attenuator to the sensitivity of Infector Detector.
Having little LuxR present, will form very little binding complex with AHL and thus the sensitivity will decrease significantly. Saturating the detection compound with LuxR will maximise the sensitivity.
Packaging
Infector Detector can be packaged as either a cream or a spray.
A spray will provide easy application of the detector because it does not require the user to fiddle around with the urinary catheter as they can simply spray from a distance. The disadvantage is the poor accuracy of application, waste, and higher rate of evaporation.
A cream, on the other hand, will decrease significantly any evaporation and will allow the user to apply the detector to specific areas of the catheter with more control. The disadvantage is that the diffusion rate of AHL and detection compounds through a viscous cream is lower. This will slow down system response.
Both applications provide some advantages and disadvantages that must be weighed depending on the actual use scenario of Infector Detector in order to decide which is best.
As you can see, the development of the application can easily be extended into many areas, and even though it is far from being commercially available, Infector Detector has proven that even the tiniest of members of the microcosm around us can tackle a problem of worldwide dimensions.
References
- Damien Balestrino et al. Characterization of Type 2 Quorum Sensing in Klebsiella pneumoniae and Relationship with Biofilm Formation. J Bacteriol. 2005 April; 187(8): 2870–2880.