Imperial/Wet Lab/Results/CBD2.1
From 2007.igem.org
m (→'''20°C''') |
(→Discussion) |
||
| (18 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | {{Template: IC07navmenu}} | ||
| + | <br clear="all"> | ||
__NOTOC__ | __NOTOC__ | ||
| - | |||
| - | = | + | =Wet Lab: ResultsTest for optimum packaging and sampling techniques= |
| + | |||
| + | |||
==Aim== | ==Aim== | ||
| - | + | *Usage of Construct [http://partsregistry.org/Part:BBa_I13522 '''pTet-GFP'''] | |
| - | <br>''' | + | *Determine an optimum packaging technique for the experiments, that minimises the evaporation of the reaction mixture. |
| + | *Determine a suitable sampling technique which disrupts the reaction to a very minimum | ||
| + | <br>'''07-10-2007''' | ||
| - | == | + | ==Materials and Methods== |
| - | + | See [[Imperial/Wet_Lab/Protocols/CBD2.1|Protocols]] page | |
| + | <br> | ||
| - | + | ==Results== | |
| - | {| | + | {|align="center" |
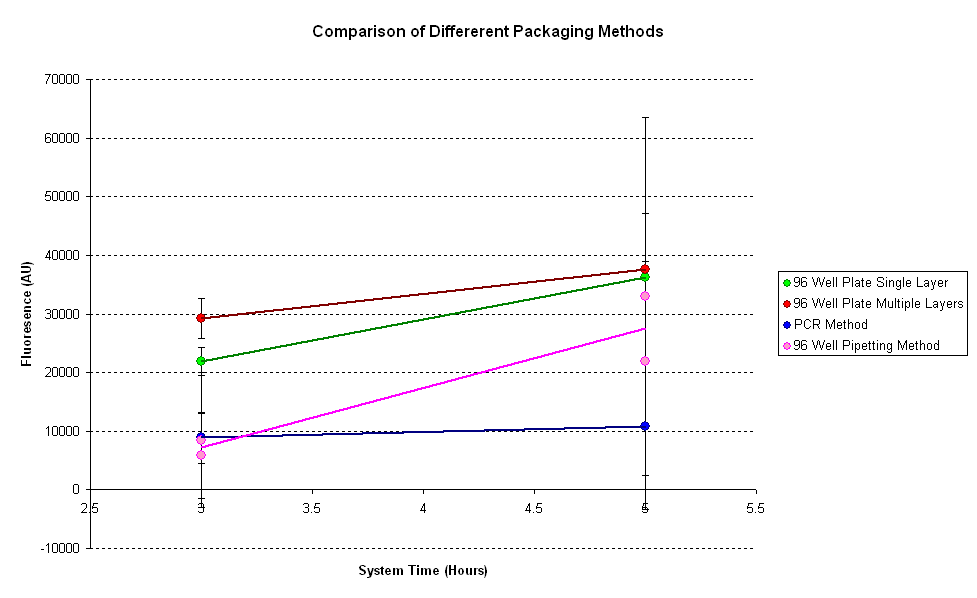
| - | | [[ | + | | width="600px"|<br>[[Image:IC 2007 packaging.PNG|thumb|600px|Fig.1.1: '''pTet-GFPmut3b''' - fluorescence output for various packaging and sampling techniques, over a period of 6 hours]] |
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | + | '''Controls:''' | |
| - | + | *Negative Control - 96 well plate with singl layered tape and no pipetting (same experimental conditions as have them usually) | |
| - | + | ||
| - | + | '''Constants:''' | |
| - | + | *Temperature - 37°C | |
| - | + | *Volume of Cells sampled - 60µl | |
| + | |||
| + | |||
| + | '''Raw Data''' | ||
| + | *[[Media:IC 2007 CBD Packaging.xls|Raw Data excel file]] | ||
| + | |||
| + | ==Discussion== | ||
| + | It can be seen from the results that the fluorescence is the highest for the plate with multiple layers of tape. Thus putting extra tape on the wells changes the fluorescence of the system. As it has been seen before, evaporation of samples results in greater fluorescence, thus there is more evaporation in the multi-layered plate. | ||
| + | |||
| + | For the pipetting sample, two readings were taken for each data point. One before and one after pipetting the mixture. It can be seen that pipetting disrupts the reaction as fluorescence after pipetting increased to a very high value. | ||
| + | |||
| + | The PCR method seems to have the least fluorescnce out of all the samples. As PCR method requires pipetting and centrifuging (not tested here), there will be a lot of disruption in the reaction. | ||
| + | |||
| + | ==Conclusion== | ||
| + | It can be concluded from the above results that it is not possible to carry out experiments over a time period greater than one day (about 9 hours of lab). This is as it is not possible to reduce evaporation so it is not signifcant over a few days. Having a stock solution of the reaction mixture is also not feasible as the cell extract is very expensive. | ||
Latest revision as of 03:00, 27 October 2007

Wet Lab: ResultsTest for optimum packaging and sampling techniques
Aim
- Usage of Construct [http://partsregistry.org/Part:BBa_I13522 pTet-GFP]
- Determine an optimum packaging technique for the experiments, that minimises the evaporation of the reaction mixture.
- Determine a suitable sampling technique which disrupts the reaction to a very minimum
07-10-2007
Materials and Methods
See Protocols page
Results
Controls:
- Negative Control - 96 well plate with singl layered tape and no pipetting (same experimental conditions as have them usually)
Constants:
- Temperature - 37°C
- Volume of Cells sampled - 60µl
Raw Data
Discussion
It can be seen from the results that the fluorescence is the highest for the plate with multiple layers of tape. Thus putting extra tape on the wells changes the fluorescence of the system. As it has been seen before, evaporation of samples results in greater fluorescence, thus there is more evaporation in the multi-layered plate.
For the pipetting sample, two readings were taken for each data point. One before and one after pipetting the mixture. It can be seen that pipetting disrupts the reaction as fluorescence after pipetting increased to a very high value.
The PCR method seems to have the least fluorescnce out of all the samples. As PCR method requires pipetting and centrifuging (not tested here), there will be a lot of disruption in the reaction.
Conclusion
It can be concluded from the above results that it is not possible to carry out experiments over a time period greater than one day (about 9 hours of lab). This is as it is not possible to reduce evaporation so it is not signifcant over a few days. Having a stock solution of the reaction mixture is also not feasible as the cell extract is very expensive.