Imperial/Wet Lab/Results/ID2.1
From 2007.igem.org
m |
(→Discussion) |
||
| (4 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Template: IC07navmenu}} | {{Template: IC07navmenu}} | ||
| + | <br clear="all"> | ||
__NOTOC__ | __NOTOC__ | ||
| Line 10: | Line 11: | ||
Refer to [[Imperial/Wet_Lab/Protocols/ID2.1|Protocol]] page. | Refer to [[Imperial/Wet_Lab/Protocols/ID2.1|Protocol]] page. | ||
<br> | <br> | ||
| - | |||
==Results== | ==Results== | ||
| Line 37: | Line 37: | ||
Another interesting observation is that the 6µg of DNA begins producing GFPmut3b greater than 2µg but then levels of before 2µg. This is thought to be because the data displayed on the figures are averages of 2 samples, one of the samples levels off sooner than the other and so brings the average down. However, the results do tell us that 6µg is lower than the 4µg, therefore the 4µg is the optimum DNA for our ''in vitro'' chassis. | Another interesting observation is that the 6µg of DNA begins producing GFPmut3b greater than 2µg but then levels of before 2µg. This is thought to be because the data displayed on the figures are averages of 2 samples, one of the samples levels off sooner than the other and so brings the average down. However, the results do tell us that 6µg is lower than the 4µg, therefore the 4µg is the optimum DNA for our ''in vitro'' chassis. | ||
| - | This agrees with promegas guide on using the commcerial cell extract ''in vitro'' chassis. The guide states that 4µg is the maximum and above this there is problems with premature termination of translational products | + | This agrees with promegas guide on using the commcerial cell extract ''in vitro'' chassis. The guide states that 4µg is the maximum and above this there is problems with premature termination of translational products. |
| + | |||
| + | When the results for this experiment are compared to a similar experiment carried out for '''pTet-GFPmut3b''', the difference in optimum DNA concentration becomes apparent. The '''pTet-LuxR-pLux-GFPmut3b''' showed an optimum at 4µg, above which the output of GFP molecules decreases. This is not the case for '''pTet-GFPmut3b'''. Although there is no increase above 4µg, there is also no decrease. | ||
| + | |||
| + | Promega states that the optimum for the commercial cell extract is 4µg, above which there is a decrease in protein synthesis because of premature termination of translation. The reason for the difference in optimum DNA concentration must be in the constructs used. '''pTet-LuxR-pLux-GFPmut3b''' is a large construct that produces two proteins; LuxR and GFPmut3b. In contrast the '''pTet-GFPmut3b''' is a smaller construct that only produces GFPmut3b. Hence when DNA concentration of '''pTet-LuxR-pLux-GFPmut3b''' increases, more energy of the system is spent on production of the LuxR protein rather than synthesis of GFP; this is also due to the fact that pTet is a strong promoter, and induces high transcription rate of LuxR. | ||
==Conclusion== | ==Conclusion== | ||
To conclude the following approximations can be made:<br> | To conclude the following approximations can be made:<br> | ||
*Optimum DNA concentrations - 4µg of DNA | *Optimum DNA concentrations - 4µg of DNA | ||
Latest revision as of 03:15, 27 October 2007

Testing DNA concentration of pTet-LuxR-pLux-GFPmut3b In vitro
Aims
To determine the optimum concentration of [http://partsregistry.org/Part:BBa_T9002 pTet-LuxR-pLux-GFPmut3b] in vitro.
Materials and Methods
Refer to Protocol page.
Results
Controls:
- Negative Control- Nuclease Free Water was added instead of DNA
Constants:
- Temperature - 25°C
- Total Volume - 60µl
Raw Data
Discussion
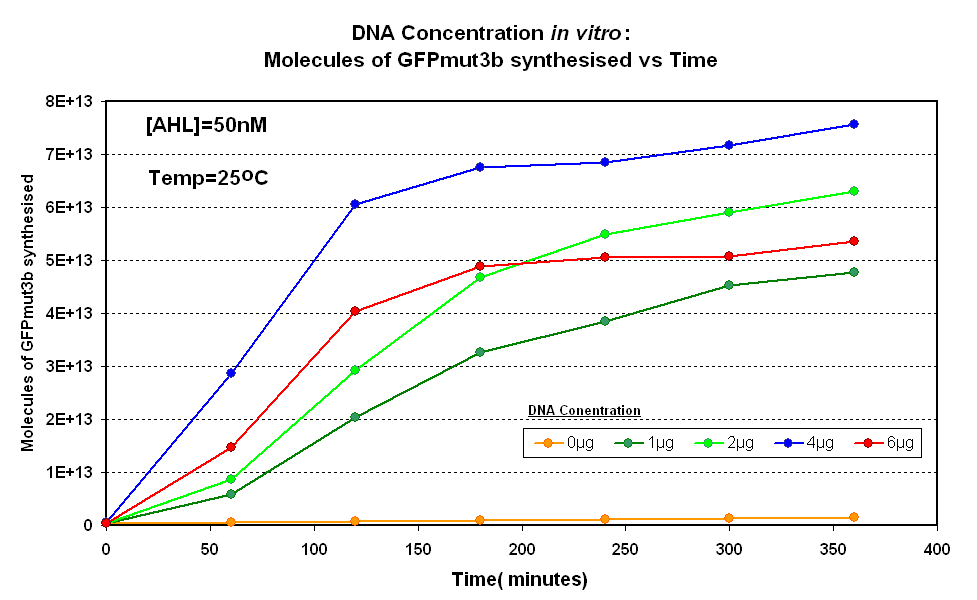
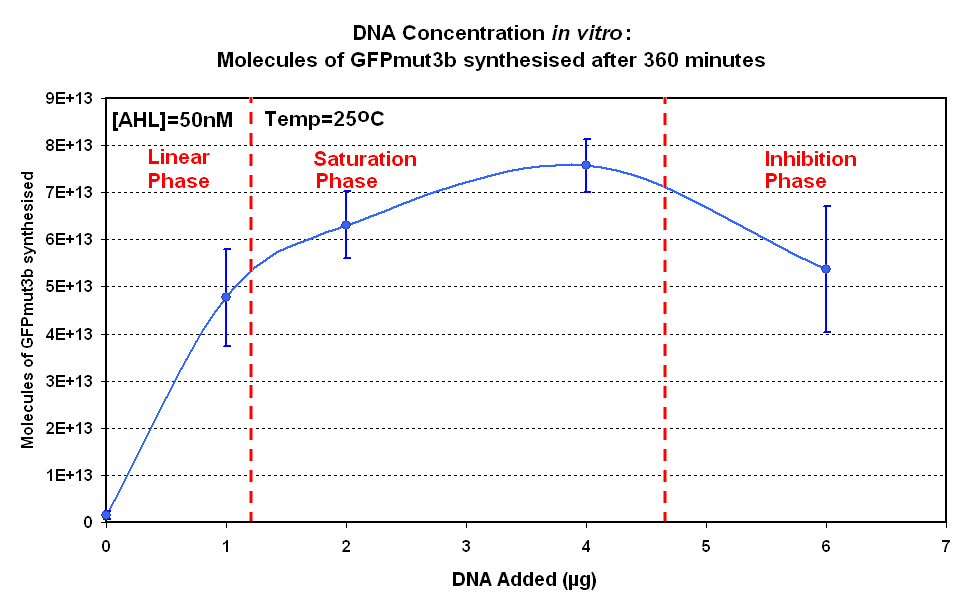
Figure 1.1 and Figure 1.2. shows us that the synthesis of GFPmut3b increases with DNA added up until 4µg of DNA. Above this the number of molecules of GFPmut3b produced decreases.
Another interesting observation is that the 6µg of DNA begins producing GFPmut3b greater than 2µg but then levels of before 2µg. This is thought to be because the data displayed on the figures are averages of 2 samples, one of the samples levels off sooner than the other and so brings the average down. However, the results do tell us that 6µg is lower than the 4µg, therefore the 4µg is the optimum DNA for our in vitro chassis.
This agrees with promegas guide on using the commcerial cell extract in vitro chassis. The guide states that 4µg is the maximum and above this there is problems with premature termination of translational products.
When the results for this experiment are compared to a similar experiment carried out for pTet-GFPmut3b, the difference in optimum DNA concentration becomes apparent. The pTet-LuxR-pLux-GFPmut3b showed an optimum at 4µg, above which the output of GFP molecules decreases. This is not the case for pTet-GFPmut3b. Although there is no increase above 4µg, there is also no decrease.
Promega states that the optimum for the commercial cell extract is 4µg, above which there is a decrease in protein synthesis because of premature termination of translation. The reason for the difference in optimum DNA concentration must be in the constructs used. pTet-LuxR-pLux-GFPmut3b is a large construct that produces two proteins; LuxR and GFPmut3b. In contrast the pTet-GFPmut3b is a smaller construct that only produces GFPmut3b. Hence when DNA concentration of pTet-LuxR-pLux-GFPmut3b increases, more energy of the system is spent on production of the LuxR protein rather than synthesis of GFP; this is also due to the fact that pTet is a strong promoter, and induces high transcription rate of LuxR.
Conclusion
To conclude the following approximations can be made:
- Optimum DNA concentrations - 4µg of DNA