Imperial/Wet Lab/Results/Res1.5
From 2007.igem.org
(→Conclusion) |
m |
||
| (3 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Template:IC07navmenu}} | {{Template:IC07navmenu}} | ||
| + | <br clear="all"> | ||
__NOTOC__ | __NOTOC__ | ||
| Line 8: | Line 9: | ||
__NOTOC__ | __NOTOC__ | ||
==Aims== | ==Aims== | ||
| - | To purifiy LuxR protein for the | + | To purifiy LuxR protein for the Infector Detector construct 2. Using purified protein will mean that a the LuxR will not have to be synthesized by the ''in vitro'' chassis and so potentially will synthesize a greater number of GFPmut3b molecules. In addition, the modelling of construct 2 tells us that the response of synthesis of GFPmut3b will be much quicker. |
==Materials and Methods== | ==Materials and Methods== | ||
| Line 15: | Line 16: | ||
==Results== | ==Results== | ||
{|align="center" | {|align="center" | ||
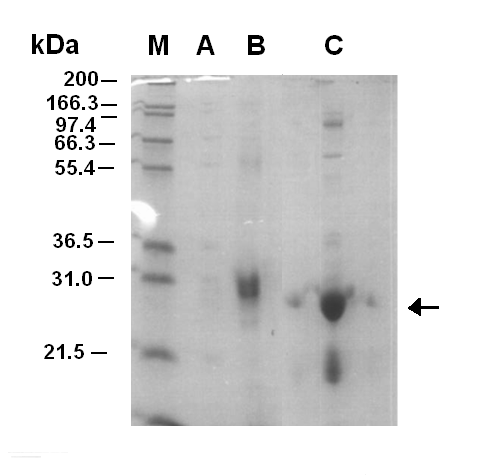
| - | | width="400px"|<br>[[Image:Purification of LuxR.PNG|thumb|300px|Figure 1.1. SDS-PAGE Gel (12%) of purification fractions. The picture is of an SDS-PAGE Gel of the fractions from the | + | | width="400px"|<br>[[Image:Purification of LuxR.PNG|thumb|300px|'''Figure 1.1. SDS-PAGE Gel (12%) of purification fractions.''' The picture is of an SDS-PAGE Gel of the fractions from the nickel affinity column. 2ul of fractions were laoded with 8ul water and 2ul of loading buffer. Sample M is a marker, Sample A is the fraction run off and Sample B and C are fractions 6 and 7 respectivly. It can be seen that fraction C contains a large band around 28kDa which corresponds to the LuxR protein.]] |
|} | |} | ||
==Discussion== | ==Discussion== | ||
| - | Figure 1.1 shows that the purificatin of LuxR was successful with fraction 7 containing the LuxR protein. After the fractionation the sample was concentrated | + | Figure 1.1 shows that the purificatin of LuxR was successful with fraction 7 containing the LuxR protein. After the fractionation the sample was concentrated using a molecular filter. However, the first time this was done the sample of LuxR was lost. When the purification was carried out for a second time the LuxR was not lost during concentration, but precipitate began to form. This was thought to be the LuxR protein coming out of solubility. Later on the LuxR protein was tested for functionality, it was shown that it was non-functional. |
==Conclusion== | ==Conclusion== | ||
The LuxR protein was successfully purified, however the protein lost functionaility in the fractionation. The reason for this is thought to be that the protein was over expressed and isolated from the insoluble proteins/inclusion bodies of the cell. We attempted to refold the protein, but failed as it did not fold correctly or was unstable in the folded form. | The LuxR protein was successfully purified, however the protein lost functionaility in the fractionation. The reason for this is thought to be that the protein was over expressed and isolated from the insoluble proteins/inclusion bodies of the cell. We attempted to refold the protein, but failed as it did not fold correctly or was unstable in the folded form. | ||
Latest revision as of 02:10, 27 October 2007

Purification of LuxR
Aims
To purifiy LuxR protein for the Infector Detector construct 2. Using purified protein will mean that a the LuxR will not have to be synthesized by the in vitro chassis and so potentially will synthesize a greater number of GFPmut3b molecules. In addition, the modelling of construct 2 tells us that the response of synthesis of GFPmut3b will be much quicker.
Materials and Methods
Link to the Protocol
Results
Discussion
Figure 1.1 shows that the purificatin of LuxR was successful with fraction 7 containing the LuxR protein. After the fractionation the sample was concentrated using a molecular filter. However, the first time this was done the sample of LuxR was lost. When the purification was carried out for a second time the LuxR was not lost during concentration, but precipitate began to form. This was thought to be the LuxR protein coming out of solubility. Later on the LuxR protein was tested for functionality, it was shown that it was non-functional.
Conclusion
The LuxR protein was successfully purified, however the protein lost functionaility in the fractionation. The reason for this is thought to be that the protein was over expressed and isolated from the insoluble proteins/inclusion bodies of the cell. We attempted to refold the protein, but failed as it did not fold correctly or was unstable in the folded form.