Imperial/Infector Detector/F2620 Comparison
From 2007.igem.org
m (→Summary of Comparison) |
(→Summary of Comparison) |
||
| Line 70: | Line 70: | ||
<span style="font-size:120%;">'''Key Difference:'''</span> | <span style="font-size:120%;">'''Key Difference:'''</span> | ||
| - | *'''Shape of Transfer Function'''- a sigmoidal shaped transfer function with a step linear region flanked by a lower threshold and upper threshold is seen for both chassis. This | + | *'''Shape of Transfer Function'''- a sigmoidal shaped transfer function with a step linear region flanked by a lower threshold and upper threshold is seen for both chassis. This indicates that the characteristic response of the construct is independent of the chassis for a given strain i.e both of these ''in vitro'' and ''in vivo'' chassis are from ''E.coli''. |
| - | *'''Rate of GFP Synthesis''' - The rate of synthesis is greater in the ''in vitro'' chassis e.g. for 1000nM AHL the rate is 1400 GFP molecules synthesis per second per cell equivalent for ''in vitro'' and 500 GFP molecules synthesis per second per cell. This makes sense because the commercial E.coli cell extract we use is optimised for protein synthesis. | + | *'''Rate of GFP Synthesis''' - The rate of synthesis is greater in the ''in vitro'' chassis e.g. for 1000nM AHL the rate is 1400 GFP molecules synthesis per second per cell equivalent for ''in vitro'', and 500 GFP molecules synthesis per second per cell. This makes sense because the commercial E.coli cell extract we use is optimised for protein synthesis. |
*'''Upper Threshold''' - The upper threshold of response looks as if it occurs at ~1000nM for both chassis. The saturation is due to the saturation of expression equipment, meaning that the system cannot respond to any increase to AHL. | *'''Upper Threshold''' - The upper threshold of response looks as if it occurs at ~1000nM for both chassis. The saturation is due to the saturation of expression equipment, meaning that the system cannot respond to any increase to AHL. | ||
| - | *'''Lower Threshold''' - The lower threshold of response is 1nM for ''in vivo'', however because of the lack of data points we had to extrapolate this for ''in vitro''. From the shape of the ''in vitro'' data we collected it | + | *'''Lower Threshold''' - The lower threshold of response is 1nM for ''in vivo'', however because of the lack of data points we had to extrapolate this for ''in vitro''. From the shape of the ''in vitro'' data we collected, it seems the threshold could be at a higher concentration to AHL, meaning that the ''in vitro'' chassis is less sensitive to lower AHL concentrations. However without these data points it is difficult to define this threshold accurately. |
|} | |} | ||
Revision as of 00:18, 27 October 2007

Summary of Comparison
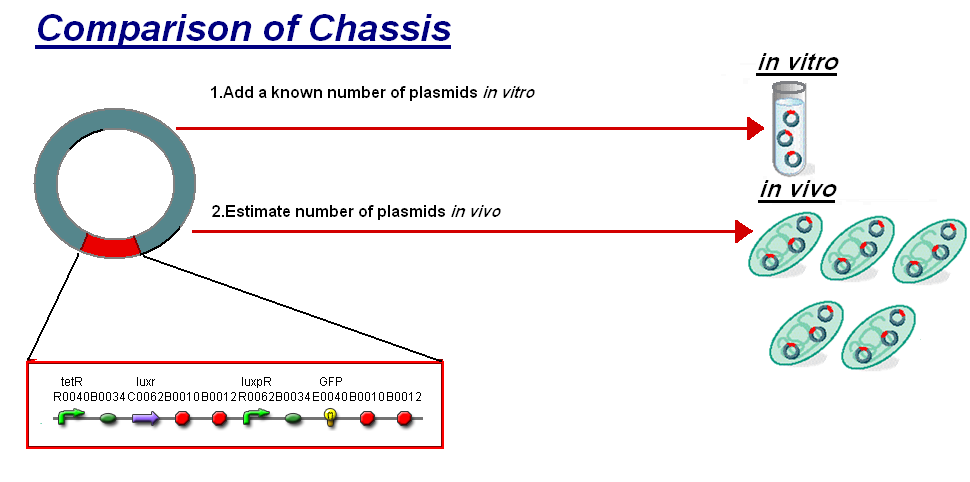
We thought to compare our in vitro characterisation to the characterisation of [http://partsregistry.org/Part:BBa_F2620 F2620] in vivo with the aim to highlight some of the differences between the chassis and investigate how the construct's characteristics may change between them. The [http://partsregistry.org/Part:BBa_F2620 F2620] is an ideal construct for comparison because of its detailed characterisation in vivo. The construct is the same as the construct 1 that was used for infecter detector, pTet-LuxR-pLux-GFPmut3b. The key results of the comparison were:
- The creation of a new unit to allow comparison between in vitro and in vivo chassis.
- That although we are changing the E.coli chassis from in vivo to in vitro the construct's characteristic response is independent of the chassis.
These are exciting findings, revealing the potential for the exploration of new chassis and the ability to use constructs in an exchangable manner.
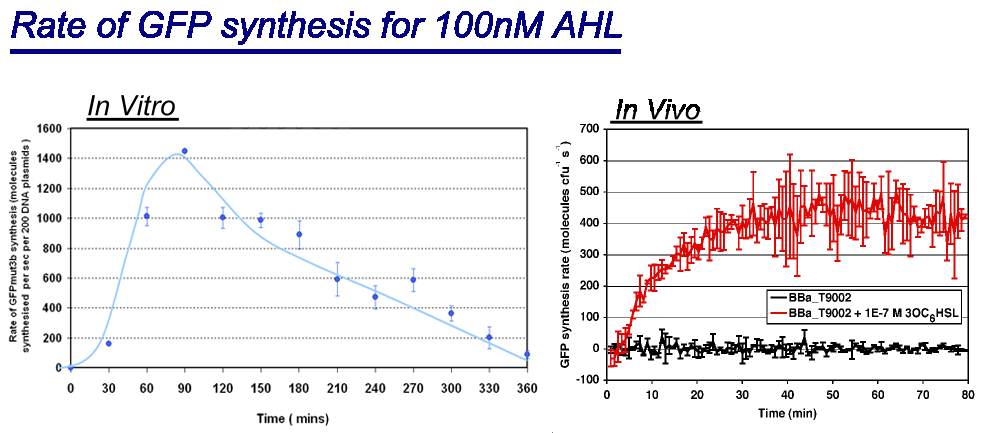 |
| Comparison between in vivo and in vitro for rate of GFPmut3b synthesis for 100nM AHL. The in vivo chassis used was the bacterial strain MG1655 and the in vitro chassis was Promega Commercial S30 Cell Extract(60µl)
Key Difference:
Interestingly the values of rate of synthesis are in the same order magnitude of hundreds, this suggesting that the normalisation we are using to compare these chassis is valid. |
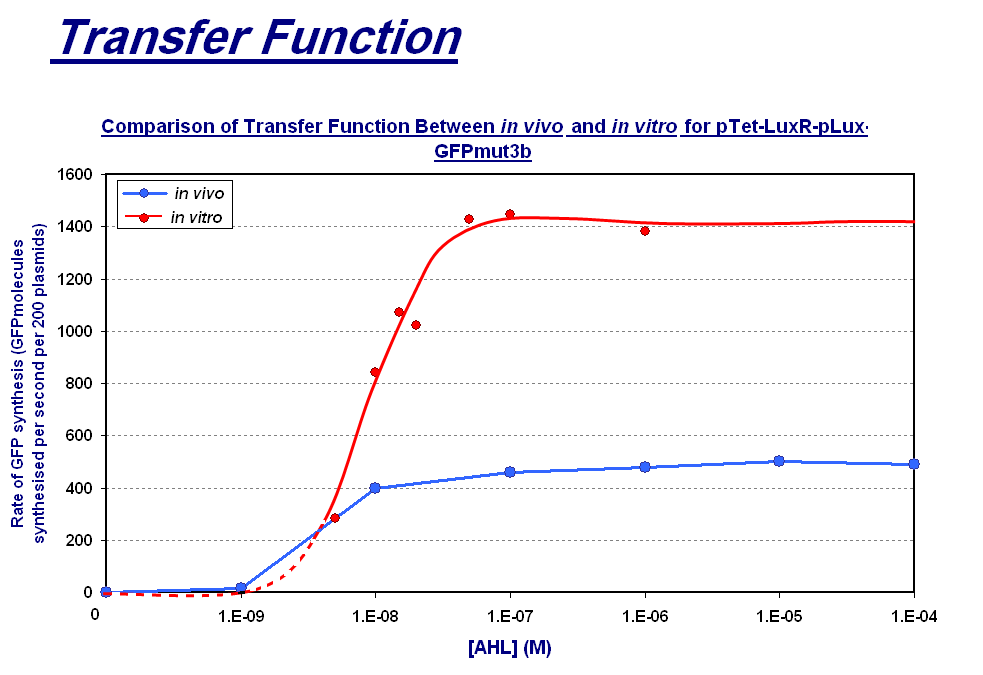 |
The graph above shows the transfer function of [AHL] input vs rate of GFP synthesis output. The graph shows the follwoing:
|