Imperial/Infector Detector/Testing
From 2007.igem.org
(→AHL Testing) |
m (→DNA Concentrations) |
||
| Line 34: | Line 34: | ||
<br clear=all> | <br clear=all> | ||
{|align="center" style="text-align: center; border-top:1px solid #000077; border-right:1px solid #000077; border-bottom:1px solid #000077; border-left:1px solid #000077;" | {|align="center" style="text-align: center; border-top:1px solid #000077; border-right:1px solid #000077; border-bottom:1px solid #000077; border-left:1px solid #000077;" | ||
| - | |<br><center>[[Image:IC 2007 DNA Concentration.PNG|thumb|420px|left|Fig.1.1:Molecules of GFPmut3b synthesised over time, for each DNA Concentration ''in vitro'' - The fluorescence was measured over time for each experiment and converted into molecules of GFPmut3b ''in vitro'' | + | |<br><center>[[Image:IC 2007 DNA Concentration.PNG|thumb|420px|left|Fig.1.1:Molecules of GFPmut3b synthesised over time, for each DNA Concentration ''in vitro'' at 25<sup>o</sup>C - The fluorescence was measured over time for each experiment and converted into molecules of GFPmut3b ''in vitro'' |
[[Imperial/Wet Lab/Results/Res1.3/Converting_Units| using our calibration curve]] Click here for [[Imperial/Wet_Lab/Results/ID2.1| results]] and [[Imperial/Wet_Lab/Protocols/ID2.1| protocol]].]] | [[Imperial/Wet Lab/Results/Res1.3/Converting_Units| using our calibration curve]] Click here for [[Imperial/Wet_Lab/Results/ID2.1| results]] and [[Imperial/Wet_Lab/Protocols/ID2.1| protocol]].]] | ||
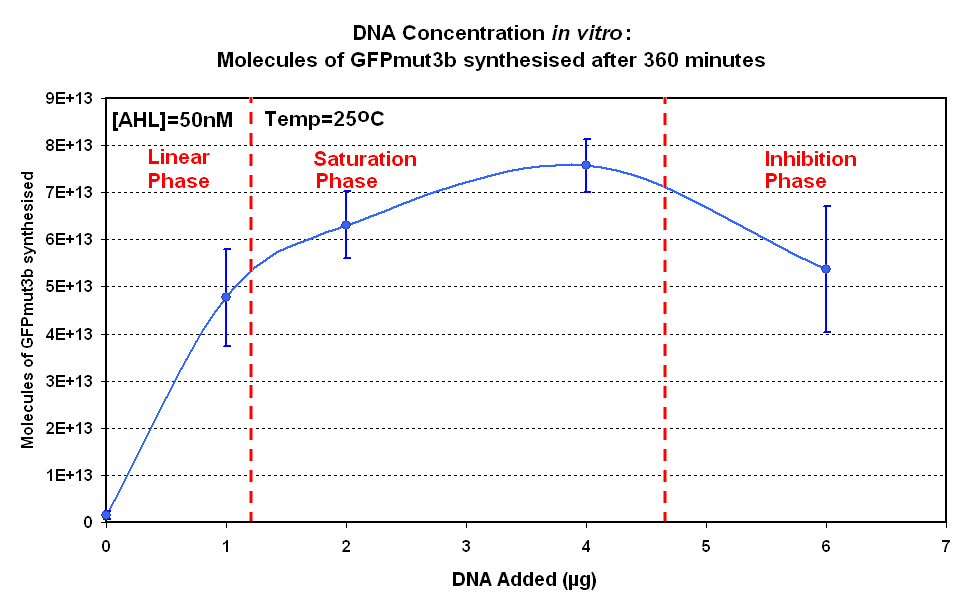
| - | [[image:IC 2007 DNA Concentration 360mins.PNG|thumb|420px|right|Fig.1.2:Molecules of GFPmut3b synthesised for each DNA Concentration ''in vitro'', after 360 minutes.<br><br><br>]] </center> | + | [[image:IC 2007 DNA Concentration 360mins.PNG|thumb|420px|right|Fig.1.2:Molecules of GFPmut3b synthesised for each DNA Concentration ''in vitro'', after 360 minutes at 25<sup>o</sup>C.<br><br><br>]] </center> |
|- | |- | ||
|style="text-align: left;" |<br>The Results above show that the optimum DNA concentration for ''in vitro'' is 4µg for 50nM AHL. From figure 1.1. and 1.2 it can be seen that as DNA concentration increases above 4µg the GFPmut3b molecules synthesised decrease. Interestingly for figure 1.2 the graph can be split into several regions of how the DNA concentration changes the output of GFPmut3b synthesis: | |style="text-align: left;" |<br>The Results above show that the optimum DNA concentration for ''in vitro'' is 4µg for 50nM AHL. From figure 1.1. and 1.2 it can be seen that as DNA concentration increases above 4µg the GFPmut3b molecules synthesised decrease. Interestingly for figure 1.2 the graph can be split into several regions of how the DNA concentration changes the output of GFPmut3b synthesis: | ||
Revision as of 01:09, 27 October 2007

Infector Detector: Testing
Summary
The key results of the testing were:
- The optimum DNA concentration for [http://partsregistry.org/Part:BBa_T9002 pTet-LuxR-pLux-GFPmut3b] in our Commcercial S30 Cell extract is 4µg.
Aims
From the initial testing we determined that the construct worked in both in vivo and in vitro. Now we were concerned with:
- Optimization - To test and obtain the optimal DNA concentration for construct 1 in vitro to reach the full potential of our infecter detector system.
- Test our specifications - To test the range 5-50nM AHL defined in our specifications and characterise the output of GFPmut3b for a range of AHL inputs. We aimed to measure fluorescence and using our calibration curve convert to molecules of GFPmut3b, click here for our calibration curve and for how we used it to convert
Results
DNA Concentrations
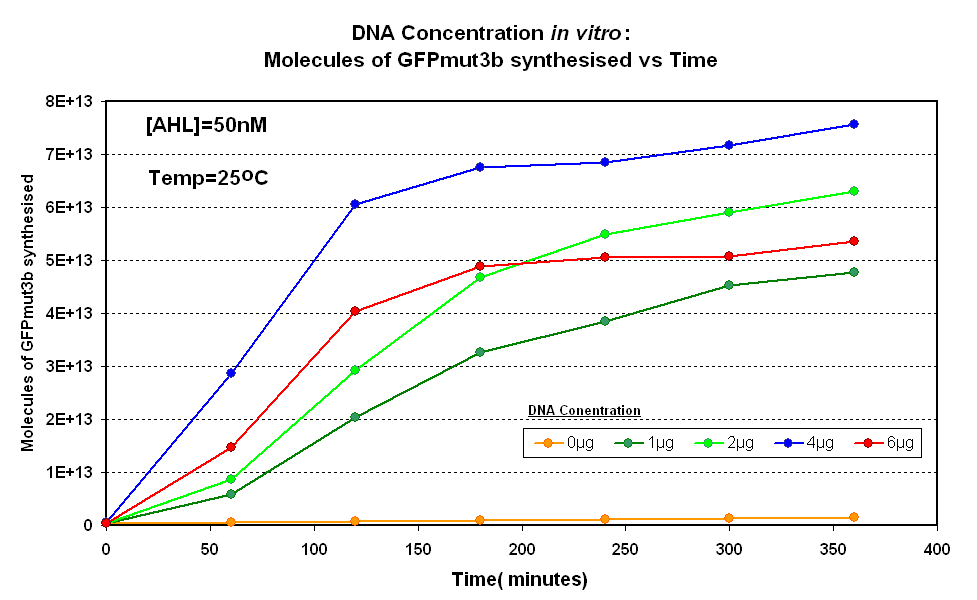 Fig.1.1:Molecules of GFPmut3b synthesised over time, for each DNA Concentration in vitro at 25oC - The fluorescence was measured over time for each experiment and converted into molecules of GFPmut3b in vitro using our calibration curve Click here for results and protocol. |
The Results above show that the optimum DNA concentration for in vitro is 4µg for 50nM AHL. From figure 1.1. and 1.2 it can be seen that as DNA concentration increases above 4µg the GFPmut3b molecules synthesised decrease. Interestingly for figure 1.2 the graph can be split into several regions of how the DNA concentration changes the output of GFPmut3b synthesis:
The fact that increasing DNA concentration above 4µg causes a decrease in rate of protein synthesis is very interesting. The reason for this is thought to be that increasing DNA concentration causes problems with premature translational termination. |
AHL Testing
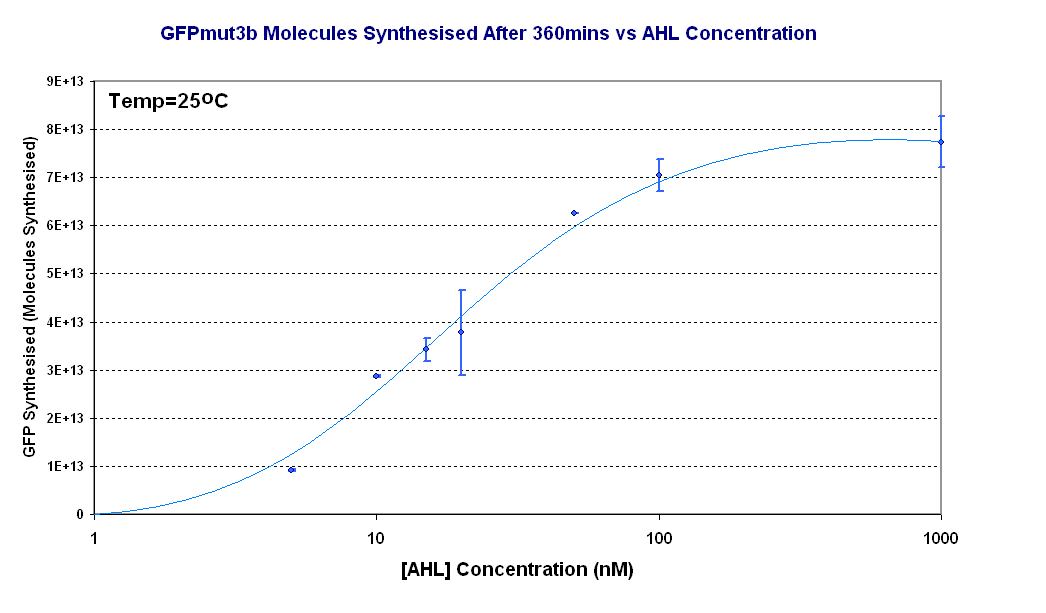
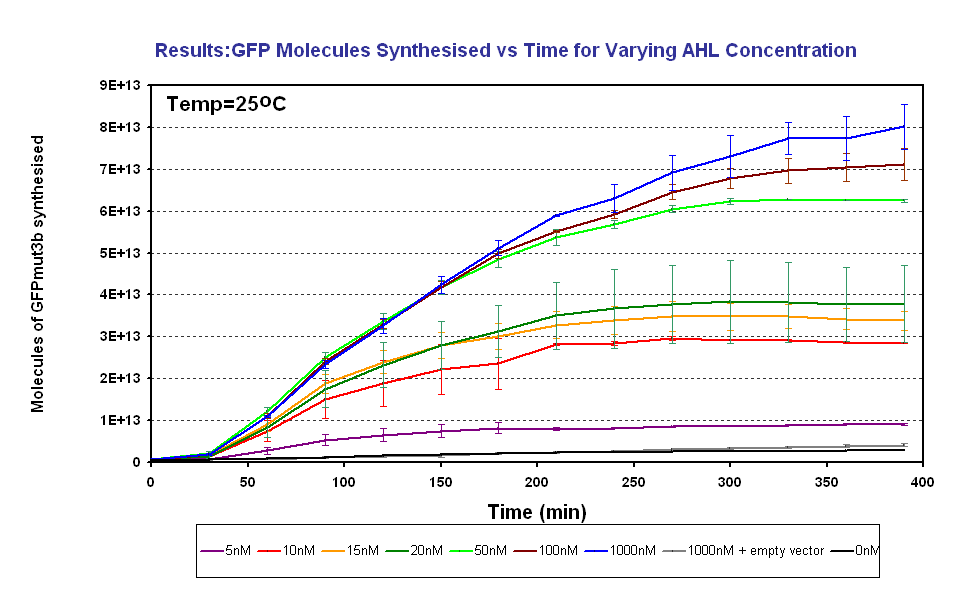 Fig.1.3: Molcules of GFPmut3b synthesised vs AHL concentrations at 25oC. The Testing was carried out for the 4µg of DNA. Again the fluorescence reading was converted into GFPmut3b molecules via the calibration curve. Click for full results and protocols can be found on the links results and protocol pages. |
Figure 1.3 shows us the following:
Figure 1.4 shows us the following:
|