Imperial/Wet Lab/Protocols/ID1.1
From 2007.igem.org

Wet Lab: Protocols: Initial Testing for DNA Constructs in vivo
Aims
- To test to see if the DNA construct from the registry are viable. This is done in vivo.
- The constructs are pTet-LuxR-pLux-GFP.
Day 1
Equipment
- 7ml sterile tubes x4
- 1.5ml Eppendorf tube x1
- 37°C incubator
- Gilson Pipettes p1000 p200 p20
Reagents
- E.coli BL21; culture containing pTet-LuxR-pLux-GFP
- LB medium
- Ampicillin stock (50 mg/ml)
- AHL stock
Innoculation of Media
- Inoculate 10ul of stored parts in individual 2ml LB medium containing 2ul of ampicillin
- Incubate at 37°C for overnight in a shaker. (This is to get a good stock of cells for use in the experiment. After the overnight culture the cells will be in stationary phase)
Preparation of culture for AHL induction and GFP measurement From the stock culture of transformed E.coli, dilute the culture so that the OD=0.1.
Preparation of diluted series of AHL
http://partsregistry.org/wiki/images/4/4c/F2620-TF-Small.png
(Taken off [http://partsregistry.org/Part:BBa_F2620 BBa_F2620]. Results show required dilution of AHL solution)
- Add 6µl of AHL stock solution to 600µl of cell samples, to a final concentration of 1uM.
Day 2
Equipment
- 37°C incubator
- Fluorometer + PC
- Gilson pipettes 1000 and 200
- 1 Fluorometer plate (black)
- Eppendorf Tubes
- Stop watch
Reagents
- LB medium
- E.coli culture with transformed plasmid
- 10μM AHL stock
- 100μM AHL stock
- GFP stock solution
- ddH2O
Protocol
Preparation of diluted GFP standard solution
- Add 995ul of ultra pure water an eppendorf tube, together with 5ul of undiluted GFP standard solution and mix. (This gives a 200x dilution to be used as a positive control)
- Place the tube on ice till it is ready to be used.
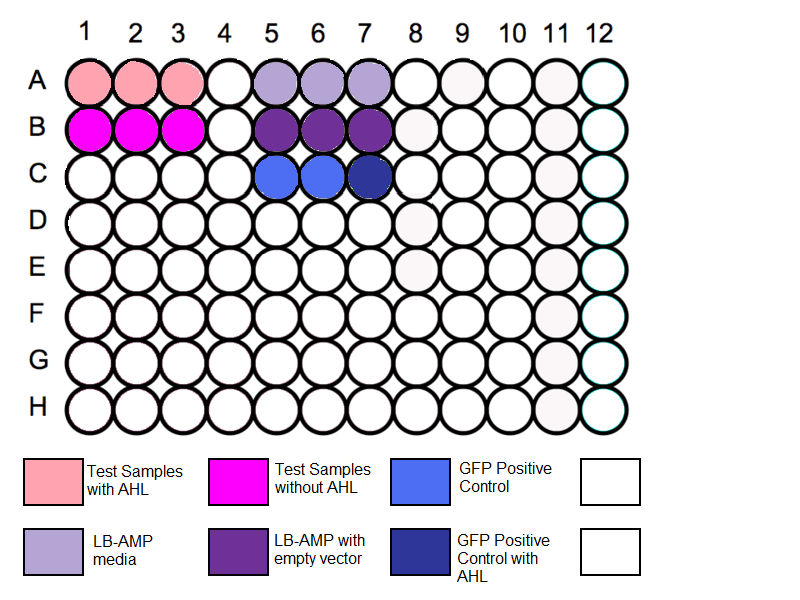
Loading Plate
1. Transfer 200 µl aliquots of each of the cultures to a flat-bottomed 96 well plate. (Follow the schematic as shown)
- Three wells to be filled with 200µl of media to measure the absorbance background.
- Three further wells to be filled with 200 µl of (media + empty-vector culture) to measure fluorescent background.
- Standard GFP solution added as a positive control.
2. Add Xµl of stock concentration of AHL to the respective wells as shown. 3. Incubate it at 37oC for 4 hours. 4. Remove lid and measure in the flourometer.
- (Fluorescence measurements - 488 nm excitation filter, 525 nm emission filter, 0.5 seconds, CW lamp energy 12901 units)
5. Repeat the measurement a further two times straight after each other (This is to test the variability of the machine)
Schematic
|