Imperial/Infector Detector/Introduction
From 2007.igem.org

Infector Detector: Introduction
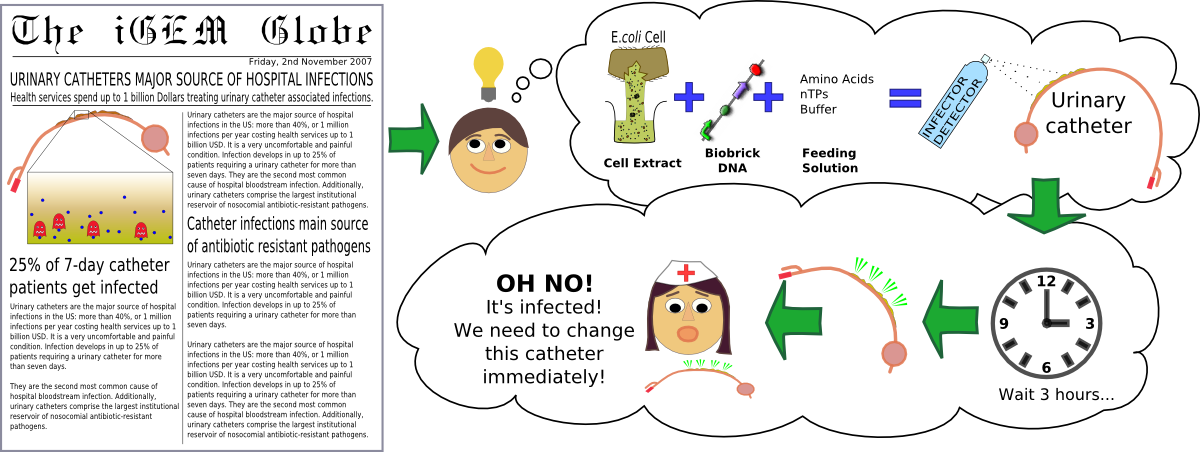
Infector Detector tackles the ongoing problem of catheter-associated urinary tract infections. To do this, we looked at how infections develop - as biofilms - and designed a system which would be able to detect their presence. We have created a system which is capable of detecting one of the types of signalling molecules found in biofilms, AHL, and visibly report its presence by producing a fluorescent protein.
Motivation
Urinary Catheter Infections
Urinary catheters are the major source of hospital infections in the US: more than 40%, or 1 million infections per year costing health services up to 1 billion USD1-4. It is a very uncomfortable and painful condition. Infection develops in up to 25% of patients requiring a urinary catheter for more than seven days5-6. They are the second most common cause of hospital bloodstream infection8-10. Additionally, urinary catheters comprise the largest institutional reservoir of nosocomial antibiotic-resistant pathogens.5-10
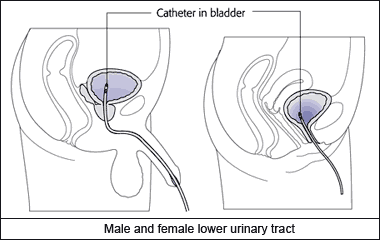
E.coli alone is responsible for over a quarter of urinary catheter infections. Pseudomonas aeruginosa is another major agent. Such infections are caused by a bacterial biofilm that forms on the surface of the catheter, and then spreads up into the urethra.
 An infected catheter12. |  Scanning electron micrograph of the surface of a catheter removed from patient with catheter-associated UTI11. |
Biofilms
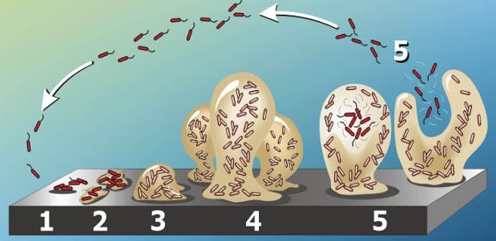
A biofilm forms when an organism attaches to a surface, and begins producing polysaccharides. This forms a protective matrix around the organism, to which other cells can attach and secrete even more polysaccharides. The biofilm grows until it begins to release cells and other debris into its environment. Released cells then attach to other parts of the surface, starting the cycle anew13-15.
And like that, the biofilm spreads around and creeps along a surface. This process takes place between a few hours to a few days.
Biofilms are very tough to eradicate - they are difficult to clean, and cells contained within are very hard to target with normal antibiotics or antiseptics.
Cell signalling inside biofilms
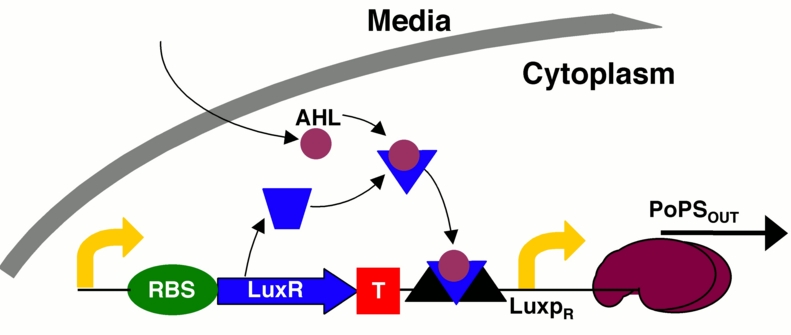
The behaviour of cells in a biofilm is very different from that of those in planktonic state. They talk to each other through molecular signalling mechanisms, such as quorum sensing and wall sensing. An important characteristic of biofilms, then, is that there are signal molecules floating around inside of it.
One type of signalling molecule, AHL, is produced by Pseudomonas aeruginosa when forming biofilms16. MRSA has a similar behaviour – the agr quorum sensing mechanism17. Every biofilm has a similar mechanism, because this type of signalling controls the growth of the biofilm.
Our system, Infector Detector, exploits exactly this mechanism in order to detect biofilms.
Achievements
- We used synthetic biology to tackle the problem of urinary catheter infections. We achieved this using only parts available in the Registry, and a new in-vitro chassis.
- We designed, built, and tested successfully a system capable of detecting AHL at concentrations as low as 5nM, and responding within 3 hours.
- We tested the system in-vivo, using E.coli, and in-vitro, using titrated AHL. The next step is to test it on real biofilms.
References
- Stamm WE. Catheter-associated urinary tract infections: Epidemiology, pathogenesis, and prevention. Am J Med 1991;91(Suppl 3B):65S-71S.
- Burke JP, Riley DK. Nosocomial urinary tract infection. In: Mayhall CG, editor. Hospital epidemiology and infection control. Baltimore: Williams and Wilkins; 1996. p. 139-53.
- Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am 1997;11:609-22.
- Kunin CM. Care of the urinary catheter. In: Urinary tract infections: detection, prevention and management. Fifth ed. Baltimore: Williams and Wilkins; 1997. p. 227-99.
- Kunin CM, McCormack RC. Prevention of catheter-induced urinary-tract infections by sterile closed drainage. N Engl J Med 1966;274:1155-61.
- Garibaldi RA, et al. An evaluation of daily bacteriologic monitoring to identify preventable episodes of catheter associated UTI. Infect Control 1982;3:466-70.
- Stark RP, Maki DG. Bacteriuria in the catheterized patient. N Engl J Med 1984;311:560-4.
- Maki DG. Nosocomial bacteremia. An epidemiologic overview. Am J Med 1981;70:719-32.
- Krieger JN, Kaiser DIL, Wenzel RP. Urinary tract etiology of bloodstream infections in hospitalized patients. J Infect Dis 1983;148:57-62.
- Bryan CS, Reynolds KL. Hospital-acquired bacteremic urinary tract infection: epidemiology and outcome. J Urol 1984,132:494-8.
- [http://www.medscape.com/viewarticle/416647_3 Bacterial Biofilms in Urology].Infect Urol 11(6):169-175, 1998.
- Laube, N. Diamonds are a Urologist's Best Friend. [http://www.biofilmsonline.com/cgi-bin/biofilmsonline/00271.html BiofilmsOnline.com] 18-Nov-2004.
- Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis 2002 Sep; 8(9) 881-90. pmid:12194761. PubMed HubMed [biofilm4]
- Donlan RM. Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis 2001 Oct 15; 33(8) 1387-92.
- Tenke P, Riedl CR, Jones GL, Williams GJ, Stickler D, and Nagy E. Bacterial biofilm formation on urologic devices and heparin coating as preventive strategy. Int J Antimicrob Agents 2004 Mar; 23 Suppl 1 S67-74. doi:10.1016/j.ijantimicag.2003.12.007
- David J. Stickler, et al. Biofilms on Indwelling Urethral Catheters Produce Quorum-Sensing Signal Molecules In Situ and In Vitro. Applied and Environmental Microbiology, September 1998, p. 3486-3490, Vol. 64, No. 9
- Manago K, et al. Biofilm formation by and accessory gene regulator typing of methicillin-resistant Staphylococcus aureus strains recovered from patients with nosocomial infections. Infect Control Hosp Epidemiol 2006 Feb; 27(2) 188-90. doi:10.1086/500620 pmid:16465637.