Imperial/Wet Lab/Results/Res1.5
From 2007.igem.org


Purification of LuxR
Aims
To purifiy LuxR protein for the infector detector construct 2. Using purified protein will mean that a the LuxR will not have to be synthesised by the in vitro chassis and so potentially will synthesis a greater number of GFPmut3b molecules. In addition, the modelling of construct 2 tells us that the response of synthesis of GFPmut3b will be much quicker.
Materials and Methods
Link to the Protocol
Results
Discussion
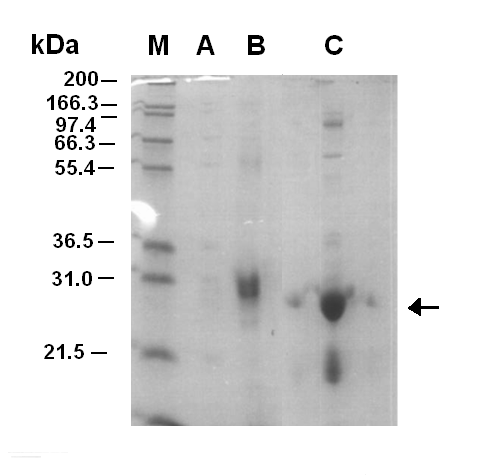
Figure 1.1 shows that the purificatin of LuxR was successful with fraction 7 containing the LuxR protein. After the fractionation the sample was concentrated up using a molecular filter. However, the first time this was done the sample of LuxR was lost. When the purification was carried out for a second time the LuxR was not lost during concentration, but precipitate did begin to form. This was thought to be the LuxR protein coming out of solubility. Later on the LuxR protein was tested for functionalility, it was shown that it was non-functional.
Conclusion
The LuxR protein was successfully purified, however the protein lost functionaility in the fractionation. The reason for this is thought to be that the protein was over expressed and isolated from the insoluble proteins/inclusion bodies of the cell. Then the LuxR protein was attempted to be refolded. Obviously either the protein did not fold correctly or was unstable in the folded form.