Imperial/Cell-Free/Characterisation
From 2007.igem.org
| Line 3: | Line 3: | ||
= Cell-Free: Characterisation = | = Cell-Free: Characterisation = | ||
| - | |||
| - | + | == Purpose == | |
| + | In this section we analyse the data we have obtained on the cell-free chassis using GFP as the reporter. We show that the classic model for the synthesis of a protein by a constitutive promoter is not adapted and introduce a new model where synthesis is curbed by the resources left in the system. Finally we estimate the parameters of this new model from our data and show that it yields a much better fit. | ||
| - | + | == Prior Knowledge == | |
| + | * pTet and pT7 are constitutive promoters that are commonly used ''in vivo'' for ''E. coli'' | ||
| + | * [[Imperial/Wet_Lab/Protocols/asdasdasf|Tests]] ''in vitro'' have demonstrated that they also work on the cell-free chassis | ||
| + | * Manufacturer specifications: the solution has been optimised so the degradation of proteins is minimal. We therefore expect our analysis to find very small degradation terms | ||
| - | + | == Available Data == | |
| - | + | We need as many data as possible and also data that are as varied as possible (data with similar behaviour will lead to the selection of a model that is too simple to predict all the behaviours that can be generated by the chassis). | |
| - | + | ||
| - | + | ||
| - | + | For this section we will use | |
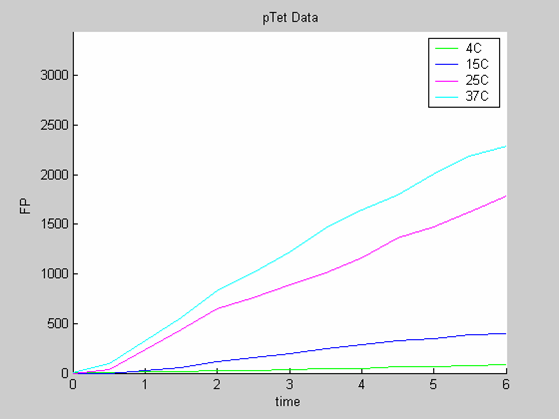
| + | * the data collected with pTet at 4,15,25 and 37 degrees (link to wiki page) that exhibit a linear behaviour | ||
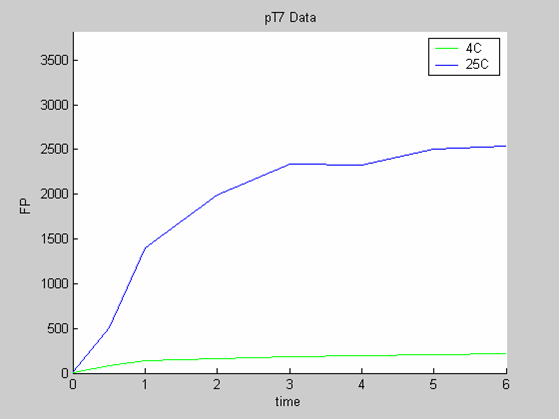
| + | * the pT7 data at 4 and 25 degrees (link to wiki page) that exhibit a saturated behaviour. | ||
| - | + | We use the calibration curve of GFP (link to wiki page) to convert the fluorescence data into concentrations (in this case the concentrations were express in nanomols/l). | |
| + | |||
| + | {| align="center" | ||
| + | |- | ||
| + | ||[[Image:IC07_CFSpTet.png|left|400px]] | ||
| + | ||[[Image:IC07_CFSpT7.png|left|400px]] | ||
| + | |} | ||
| + | ''Axes: time in hours''<br> | ||
| + | ''FP is the concentration of Fluorescent Protein (GFP here) in nanomol/l'' | ||
| + | |||
| + | |||
| + | == The Classic Model == | ||
| + | To analyse our data we use the classic approach that consists of modelling the evolution with time of the concentrations of the product of interest. | ||
| + | In this case the model is the simplest we can imagine: | ||
| + | * We have only one protein GFP | ||
| + | * It is constitutively synthesized at a constant synthesis rate K. K depends on the promoter that is used and ourdata show that it is also temperature-dependent | ||
| + | * The protein is then degraded. This degradation term δ depends on the protein and on the chassis. We can assume the impact of temperature to be negligible. | ||
| + | |||
| + | The differential equation governing the evolution of X , the concentration of GFP is | ||
| + | [[Image:IC07_CFSeqn1.png|center|100px]] | ||
| + | |||
| + | In all cases the initial concentration of GFP was X<sub>0</sub> = 0. The solution of the equation is known explicitly. It is | ||
| + | [[Image:IC07_CFSeqn2.png|center|140px]] | ||
Revision as of 23:03, 25 October 2007

Cell-Free: Characterisation
Purpose
In this section we analyse the data we have obtained on the cell-free chassis using GFP as the reporter. We show that the classic model for the synthesis of a protein by a constitutive promoter is not adapted and introduce a new model where synthesis is curbed by the resources left in the system. Finally we estimate the parameters of this new model from our data and show that it yields a much better fit.
Prior Knowledge
- pTet and pT7 are constitutive promoters that are commonly used in vivo for E. coli
- Tests in vitro have demonstrated that they also work on the cell-free chassis
- Manufacturer specifications: the solution has been optimised so the degradation of proteins is minimal. We therefore expect our analysis to find very small degradation terms
Available Data
We need as many data as possible and also data that are as varied as possible (data with similar behaviour will lead to the selection of a model that is too simple to predict all the behaviours that can be generated by the chassis).
For this section we will use
- the data collected with pTet at 4,15,25 and 37 degrees (link to wiki page) that exhibit a linear behaviour
- the pT7 data at 4 and 25 degrees (link to wiki page) that exhibit a saturated behaviour.
We use the calibration curve of GFP (link to wiki page) to convert the fluorescence data into concentrations (in this case the concentrations were express in nanomols/l).
Axes: time in hours
FP is the concentration of Fluorescent Protein (GFP here) in nanomol/l
The Classic Model
To analyse our data we use the classic approach that consists of modelling the evolution with time of the concentrations of the product of interest. In this case the model is the simplest we can imagine:
- We have only one protein GFP
- It is constitutively synthesized at a constant synthesis rate K. K depends on the promoter that is used and ourdata show that it is also temperature-dependent
- The protein is then degraded. This degradation term δ depends on the protein and on the chassis. We can assume the impact of temperature to be negligible.
The differential equation governing the evolution of X , the concentration of GFP is
In all cases the initial concentration of GFP was X0 = 0. The solution of the equation is known explicitly. It is