Imperial/Wet Lab/Protocols/Prot1.1
From 2007.igem.org

Wet Lab: Protocols: Optimisation of Fluorometer
Aims:
- To obtain an optimum counting time for the GFP fluorescence.
- Optimum counting time: Avoid photobleaching, Minimize variability between readings.
(A test to see what is the optimum counting time to have during our experiments. The counting time is the time the fluorometer detector stays on top of each well. A small window time will only account for very discrete levels of fluorescence. These might include sudden spikes of radiation since fluorescence is not a uniform process. Hence we will get variation between samples of equal expression rates. A larger counting time results in a larger window size and hence a more average reading is taken from each sample, smoothing out the variation due to the randomness of fluorescence emission.
Care must be taken however because larger counting times will lead to faster fluorescence bleaching. A compromise between the two must be found.)
Day 1
Equipment
- 7ml sterile tubes x4
- 1.5ml Eppendorf tube x1
- Room temperature 25oC
- Gilson Pipettes
Reagents
- E.coli BL21; culture containing part :pTet-GFP
- LB medium
- Ampicillin stock (50 mg/ml)
- GFP Standard Solution
Innoculation of Media
- Inoculate 10ul of transformed E.coli cells in individual 2ml LB medium containing 2ul of ampicillin.
- Incubate at 37°C overnight in a shaker. (This is to get a good stock of cells for use in the experiment. After the overnight culture the cells will be in stationary phase)
Day 2
Equipment
- Well-plate x1
- Fluorometer
- Gilson pipettes 1000 and 200
- Eppendorf tubes
Reagents
- ddH2O
- GFP standard solution
Preparation of diluted GFP standard solution
- Add 995ul of ultra pure water an eppendorf tube, together with 5ul of undiluted GFP standard solution and mix. (This gives a 200x dilution to be used as a positive control.)
- Place the tube on ice till it is ready to be used.
Loading Plate
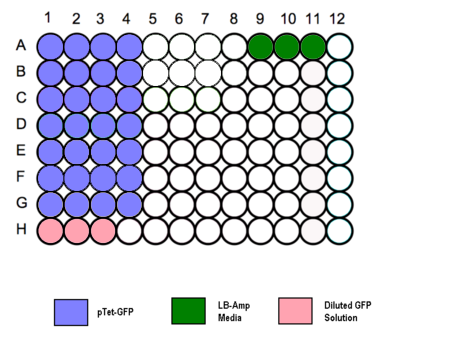
- Transfer 200 µl aliquots of each of the cultures to a flat-bottomed 96 well plate. (Follow the schematic as shown.)
- Three wells to be filled with 200µl of media to measure the absorbance background.
- Standard GFP solution added as a positive control.
- Set counting times for each well according to schematic.
- Remove lid and measure in the flourometer for 1 hour at 5 minute intervals.
- (Fluorescence measurements - 488 nm excitation filter, 525 nm emission filter, vriable counting time, CW lamp energy 5000 units)
Schematic
|