Imperial/Wet Lab/Results/CBD2.2
From 2007.igem.org
m (→Results) |
m (→Results) |
||
| (12 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Template: IC07navmenu}} | {{Template: IC07navmenu}} | ||
| + | <br clear ="all"> | ||
__NOTOC__ | __NOTOC__ | ||
| Line 11: | Line 12: | ||
==Materials and Methods== | ==Materials and Methods== | ||
Link here for [[Imperial/Wet_Lab/Protocols/CBD2.2|Protocols]] | Link here for [[Imperial/Wet_Lab/Protocols/CBD2.2|Protocols]] | ||
| + | |||
| + | |||
==Results== | ==Results== | ||
'''14-09-2007'''<br> | '''14-09-2007'''<br> | ||
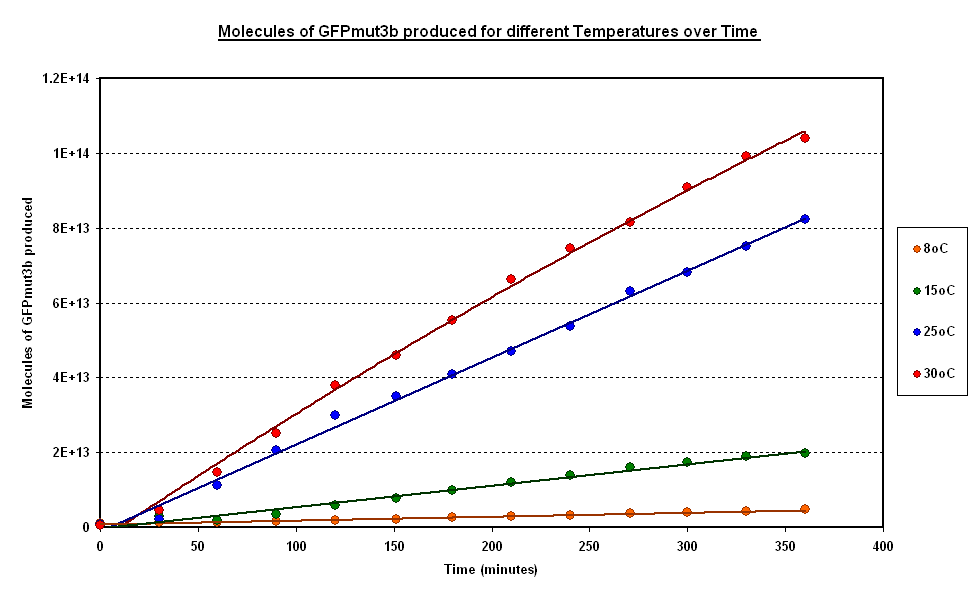
| - | The following | + | The following graph shows how the molecules of GFPmut3b produced by the pTet-GFP construct varied, on average, over time, for different temperatures. The samples were taken every 30 mins over a period of 6hours. There were three samples of cell extract with pTet-GFP DNA and one negative control for each temperature. |
| - | [[Image:CBD | + | It can be seen from Fig 1.1 that the molecules of GFPmut3b produced by te pTet-GFPmut3b has a linear relationship with the temperature. The highest number of GFPmut3b molecules are synthesized for the samples at 30°C.Full data for individual temperatures is shown below. |
| + | |||
| + | [[Image:CBD isothermal.PNG|thumb|centre|600px|Fig 1.1:Isothermal Data - Molecules of GFPmut3b produced on average by the pTet-GFPmut3b construct at different temperatures.]] <br clear="all"> | ||
<br clear="all"> | <br clear="all"> | ||
| + | |||
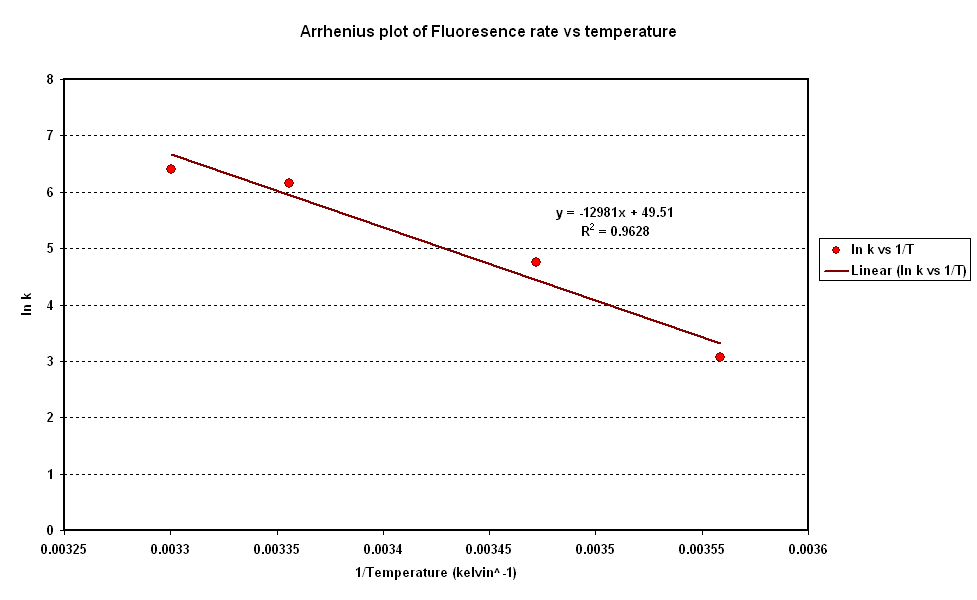
| + | Following on from the modelling phase of Cell By Date we would like to determine the activation energy of the system. We have found the activation energy of our system to be 1.5kJ/mol using the graph below. For detailed method please see excel spreadsheet below. | ||
| + | |||
| + | [[Image:CBD Arrhenius Plot.PNG|centre|thumb|600px|Fig 2: Arrhenius Plot]] <br clear = "all" > | ||
| + | {| border="2" style="background:#ABCDEF;" align = centre | ||
| + | | [[Media:CBD_Isothermal.xls|Complete set of results and raw data ]] | ||
| + | |} | ||
| + | <br clear="all"> | ||
| + | |||
| + | Methodology in Excel to yeild Activaiton Energy: | ||
| + | |||
| + | 1.Extract rates from isothermal data<br> | ||
| + | 2.Convert Temperature into Kelvin<br> | ||
| + | 3.Take natural Log of rates<br> | ||
| + | 4.Take reciprocal of Temperatures<br> | ||
| + | 5.Plot ln K vs 1/T<br> | ||
| + | 6.Linear Regression to calculate slope & R value<br> | ||
| + | 7.Division of slope by universal gas constant to yield Activation Energy<br> | ||
| + | |||
The following graphs show the average fluorescence for the different temperatures, over the three samples, at each time point. The error bars represent the standard deviation between the three samples with the DNA and the cell extract. The negative control for each temperature has also been plotted along with the average fluorescence. | The following graphs show the average fluorescence for the different temperatures, over the three samples, at each time point. The error bars represent the standard deviation between the three samples with the DNA and the cell extract. The negative control for each temperature has also been plotted along with the average fluorescence. | ||
| Line 23: | Line 47: | ||
==='''8°C'''=== | ==='''8°C'''=== | ||
[[Image:IC_2007_PTet_8oC_14-09.PNG|thumb|left|400px]] | [[Image:IC_2007_PTet_8oC_14-09.PNG|thumb|left|400px]] | ||
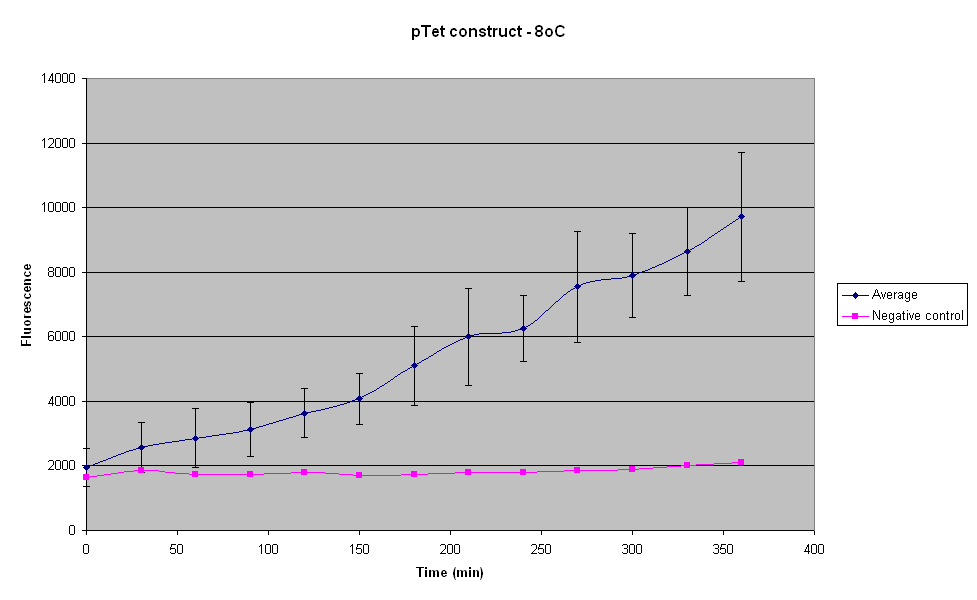
| - | The fluorescence produced by the construct seems to increase linearly over the 6 hour period. Thus the construct | + | The fluorescence produced by the construct seems to increase linearly over the 6 hour period. Thus the construct does not reach steady state at 8°C over this time period. Testing with UV light revealed that the fluorescence does not reach the visual threshold even after 6 hours. |
<br> | <br> | ||
{| border="2" style="background:#ABCDEF;" align = centre | {| border="2" style="background:#ABCDEF;" align = centre | ||
| Line 32: | Line 56: | ||
==='''15°C'''=== | ==='''15°C'''=== | ||
[[Image:IC_2007_PTet_15oC_14-09.PNG|thumb|left|400px]] | [[Image:IC_2007_PTet_15oC_14-09.PNG|thumb|left|400px]] | ||
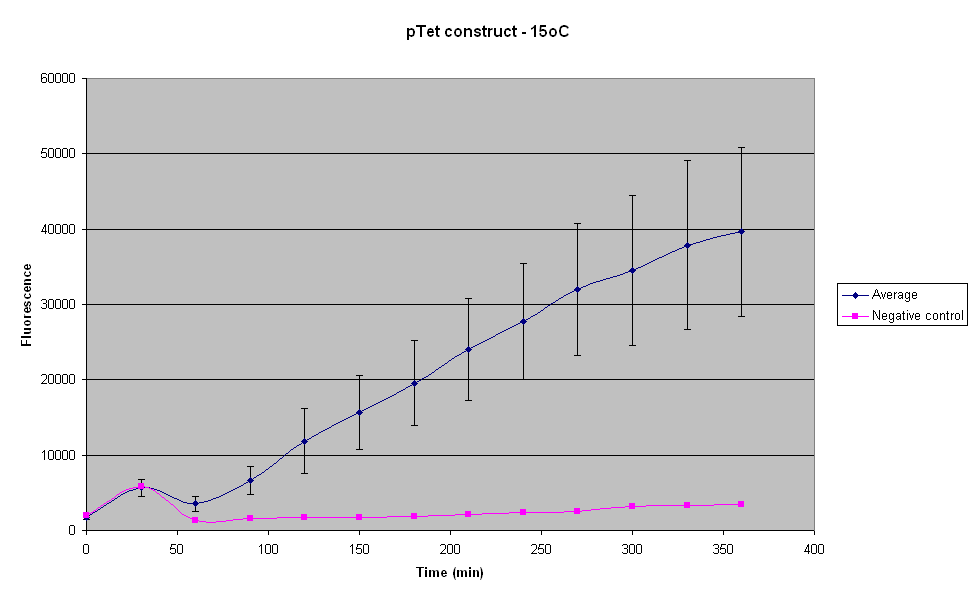
| - | The construct seems to produce negligible fluorescence for the first | + | The construct seems to produce negligible fluorescence for the first 30 mins of the reaction, if the negative control is taken into account. The maximum fluorescence is about 4 times more than that reached when the temperature is 8°C. The fluorescence was not visible at this temperature too, after 6 hours of reaction. |
| - | <br>At 30 | + | <br>At 30 min there seems to be a sudden increase in the fluorescence of the samples as well as the control. |
<br> | <br> | ||
<br> | <br> | ||
| Line 43: | Line 67: | ||
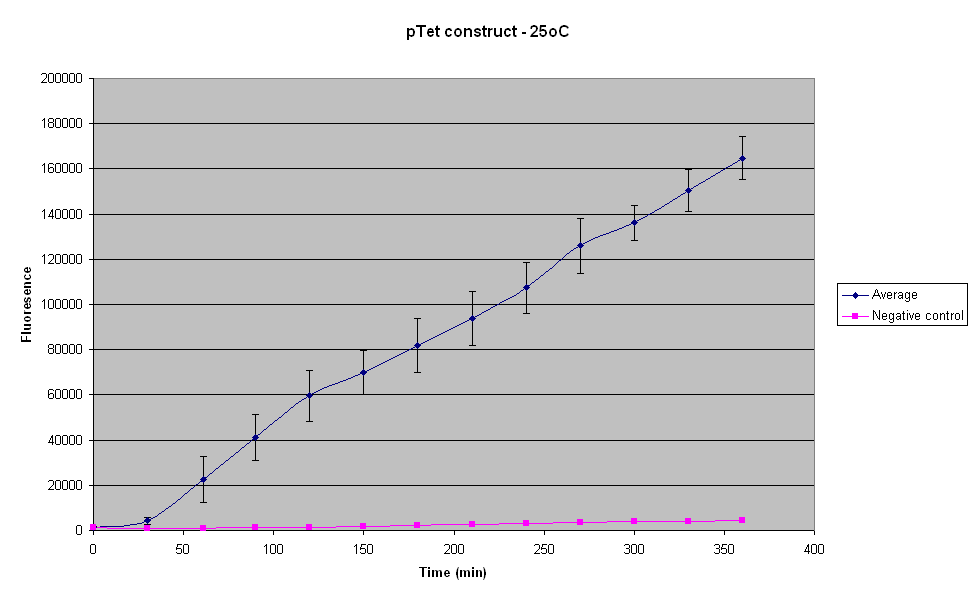
==='''25°C'''=== | ==='''25°C'''=== | ||
[[Image:IC_2007_PTet_25oC_14-09.PNG|thumb|left|400px]] | [[Image:IC_2007_PTet_25oC_14-09.PNG|thumb|left|400px]] | ||
| - | The maximum fluorescence reached by the pTet-GFP construct at 25°C is approximately 4 times greater than that reached at 15°C. The highest fluorescence is about | + | The maximum fluorescence reached by the pTet-GFP construct at 25°C is approximately 4 times greater than that reached at 15°C. The highest fluorescence is about 160 000, but even that was not visible with UV light. |
<br>The standard deviation of the fluorescence between the different samples is the lowest for 25°C compared to the other temperatures. Thus there is least variability in these results. | <br>The standard deviation of the fluorescence between the different samples is the lowest for 25°C compared to the other temperatures. Thus there is least variability in these results. | ||
<br> | <br> | ||
| Line 62: | Line 86: | ||
==Conclusion== | ==Conclusion== | ||
| - | The results show that the fluorescence produced by the pTet-GFP construct is quite a linear function of time and it increases with increasing temperature. For our applications a visual signal is very important. But after 6 hours of reaction, even with the highest fluorescence, no fluorescence could be | + | The results show that the fluorescence produced by the pTet-GFP construct is quite a linear function of time and it increases with increasing temperature. For our applications a visual signal is very important. But after 6 hours of reaction, even with the highest fluorescence, no fluorescence could be visualized with a UV light. |
Latest revision as of 03:43, 27 October 2007

Test for operation at different temperatures for pTet-GFPmut3b
Aim
The aim of these experiments was to firgure out the operating range of the [http://partsregistry.org/Part:BBa_I13522 pTet-GFP] construct in-vitro. The temperatures the construct was tested in are: 8°C, 15°C, 25°C, 30°C.
Materials and Methods
Link here for Protocols
Results
14-09-2007
The following graph shows how the molecules of GFPmut3b produced by the pTet-GFP construct varied, on average, over time, for different temperatures. The samples were taken every 30 mins over a period of 6hours. There were three samples of cell extract with pTet-GFP DNA and one negative control for each temperature.
It can be seen from Fig 1.1 that the molecules of GFPmut3b produced by te pTet-GFPmut3b has a linear relationship with the temperature. The highest number of GFPmut3b molecules are synthesized for the samples at 30°C.Full data for individual temperatures is shown below.
Following on from the modelling phase of Cell By Date we would like to determine the activation energy of the system. We have found the activation energy of our system to be 1.5kJ/mol using the graph below. For detailed method please see excel spreadsheet below.
| Complete set of results and raw data |
Methodology in Excel to yeild Activaiton Energy:
1.Extract rates from isothermal data
2.Convert Temperature into Kelvin
3.Take natural Log of rates
4.Take reciprocal of Temperatures
5.Plot ln K vs 1/T
6.Linear Regression to calculate slope & R value
7.Division of slope by universal gas constant to yield Activation Energy
The following graphs show the average fluorescence for the different temperatures, over the three samples, at each time point. The error bars represent the standard deviation between the three samples with the DNA and the cell extract. The negative control for each temperature has also been plotted along with the average fluorescence.
8°C
The fluorescence produced by the construct seems to increase linearly over the 6 hour period. Thus the construct does not reach steady state at 8°C over this time period. Testing with UV light revealed that the fluorescence does not reach the visual threshold even after 6 hours.
| Complete set of results and raw data |
15°C
The construct seems to produce negligible fluorescence for the first 30 mins of the reaction, if the negative control is taken into account. The maximum fluorescence is about 4 times more than that reached when the temperature is 8°C. The fluorescence was not visible at this temperature too, after 6 hours of reaction.
At 30 min there seems to be a sudden increase in the fluorescence of the samples as well as the control.
| Complete set of results and raw data |
25°C
The maximum fluorescence reached by the pTet-GFP construct at 25°C is approximately 4 times greater than that reached at 15°C. The highest fluorescence is about 160 000, but even that was not visible with UV light.
The standard deviation of the fluorescence between the different samples is the lowest for 25°C compared to the other temperatures. Thus there is least variability in these results.
| Complete set of results and raw data |
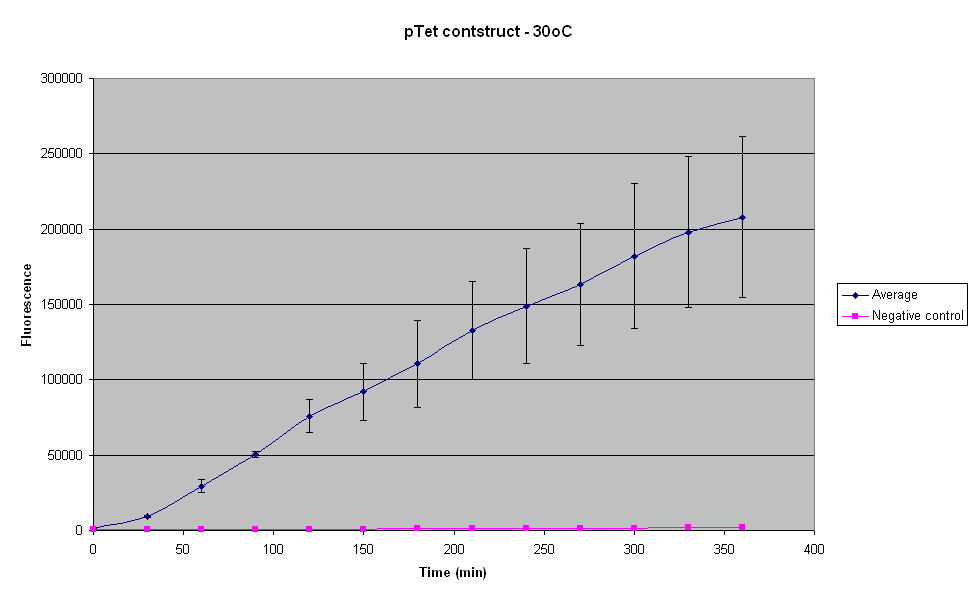
30°C
The error between the three samples seems to increase with increasing temperature. Thus the reaction becomes more and more variable as the temperature is increased above 25°C. The fluorescence from the samples at 30°C was also not visible under a UV light.
| Complete set of results and raw data |
Conclusion
The results show that the fluorescence produced by the pTet-GFP construct is quite a linear function of time and it increases with increasing temperature. For our applications a visual signal is very important. But after 6 hours of reaction, even with the highest fluorescence, no fluorescence could be visualized with a UV light.