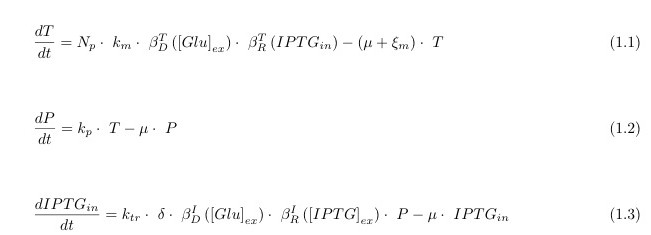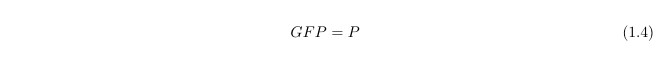Bologna
From 2007.igem.org
"Ὁ βίος βραχὺς, ἡ δὲ τέχνη μακρὴ, ὁ δὲ καιρὸς ὀξὺς, ἡ δὲ πεῖρα σφαλερὴ, ἡ δὲ κρίσις χαλεπή"
"Vita brevis, ars longa, occasio praeceps, experimentum periculosum, iudicium difficile"
"Art is long, life is short, opportunity is fleeting, experience is deceitful, judgement is difficult"
"La vita è breve, l'arte è lunga, l'occasione fuggevole, l'esperimento pericoloso, il giudizio difficile"
(Aforisma di Ippocrate di Coo)
Contents |
About Us
Welcome to Bologna’s IGEM Wiki!
Our team: Francesca Buganè, Michela Mirri, Francesco Pasqualini, and Silvia Tamarri, all undergraduate students in Biomedical Engineering; Guido Costa, undergraduate student in Electronic Engineering; Iros Barozzi, undergraduate student in Industrial Biotechnology and Bioinformatics and Alice Pasini, a PhD student in Biochemistry.
We are advised by: [http://www.ing2.unibo.it/Ingegneria+Cesena/Strumenti+del+Portale/Cerca/paginaWebDocente.htm?NRMODE=Published&TabControl1=TabContatti&UPN=emanuele.giordano%40unibo.it Dr Emanuele Giordano], Lecturer in Biochemistry and [http://www-micro.deis.unibo.it/cgi-bin/user?tartagni Prof. Marco Tartagni], Professor of Electronics. We are grateful to our advisors for their time and support!
Our instructors are: [http://www.ing2.unibo.it/Ingegneria%20Cesena/Strumenti%20del%20Portale/Cerca/paginaWebDocente?UPN=silvio.cavalcanti@unibo.it Prof. Silvio Cavalcanti], Professor of Bioengineering; Francesca Ceroni, BiotechD and [http://www-micrel.deis.unibo.it/~christine/ Christine Nardini], PhD in Electronic Engineering .
Our Project
Introduction
Our goal is the realization of a genetic circuit able to implement the functionality typical of an electronic device called Schmitt Trigger (as defined by its inventor [http://www.otto-schmitt.org/ Dr Otto H. Schmitt]).
Pardon me, what do you mean? (AKA Functional Requirements)
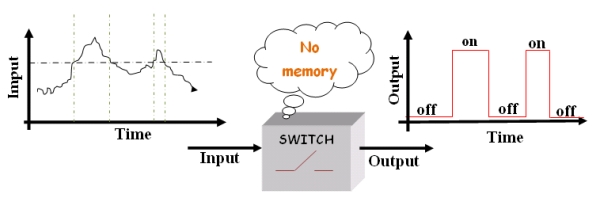
The main characteristics of this device is to be a “smart” switch: that means switch with memory.
In a "stupid" switch when the input (some environmental condition) crosses a certain threshold the output (some switch properties) changes, for instance from on to off. Often the environmental change is the modification of the value in some quantities that describes the environment (temperature, pressure, pH, ect). The "stupid" device switches just for a given value of the input (threshold).
Figure1.The "stupid" switch.
So far so good, however, if the switch input has a value that continually even minimally changes across the threshold, the device will keep going on and off, wasting energy and leaving the system in an unstable state.
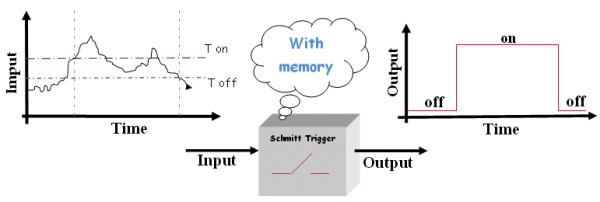
To avoid all this we need a “smart” switch. Basically, this device switches on or off at two different thresholds (High and Low thresholds called Ton and Toff respectively) depending on the history of the system. So, according to the state of the device, the threshold for switching will change.
Figure2.The "smart" switch.
This kind of "smart” switch, the "Schmitt Switch", is largely used in technical applications since it solves the instability problems, in fact, the minimal variation that causes output changes must be as large as the difference between the two thresholds. Then noise is not so critical.
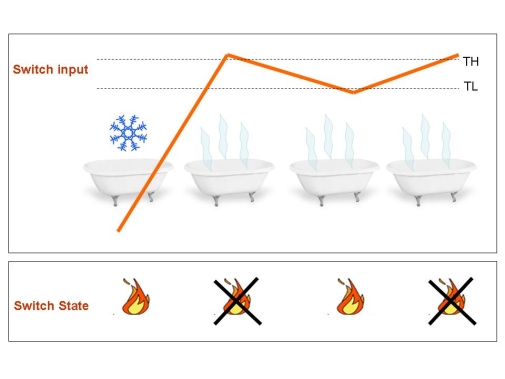
Figure 3.Typical application of the Schmitt Trigger: The boiler.Once the water temperature passes the higher threshold, boiler turn off until temperature crosses the lower threshold. Since this system work in closed loop (temperature controls the heater that determines the temperature), it is able to automatically maintain a warm temperature: the temperature holds stable and so does your mood if you are in the bath tub.
The reason why the “smart” switch works that well is due to its [http://en.wikipedia.org/wiki/Hysteresis hysteresis] properties.
Our genetic circuit tries to riproduce this fondamental property of Schmitt Trigger.
How? (AKA Technical Requirements)
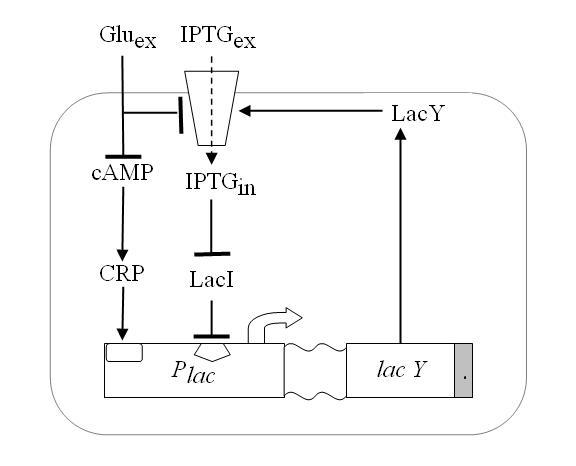
We wanted to reproduce the same principle with a genetic circuit. To do so we exploited one of such genetic systems, existing in the complex of genes that form the Lac Operon shown in Figure 4.
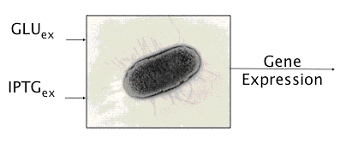
Namely, E.coli can either survive by metabolizing glucose or, in case glucose is missing, lactose. Lactose or glucose metabolism-modes are the two stable state of our system. To perform experiments we use IPTG a structural analog of lactose that cannot be metabolized. Thus the input of our system are the external concentrations of Glucose and IPTG (Gluex and IPTGex respectively).
Since lactose metabolism is more energy consuming, usually all the apparatus that takes care of the lactose metabolism is repressed. That is [http://en.wikipedia.org/wiki/Lac_operon the promoter pLac ] is constitutively repressed thank to the presence of the LacI protein that inhibits the transcription of the genes downstream of the operon ([http://bcs.whfreeman.com/mga2e/pages/bcs-main.asp?s=132&n=003&i=283&v=&o=&ns=0&uid=0&rau=0 this] is how it works). When there is IPTG in the environment of the cell, several concurrent processes take place. IPTG enters through the membrane and after some processing it is able to remove the repressor LacI, thus allowing the transcription of gene LacY that in turn enhance the IPTG uptake and increase its action on the repressor LacI. This represents the positive feedback loop we need.
Figure 4. Schematic overview of the Lac operon.
As it is the system can already perform like a Schmitt trigger, for certain values of the inputs. However there is another mechanism that allows the fine tuning of the system: glucose has a double action both on a molecule called cAMP that it bounds to, inhibiting its action on pLac expression (catabolite repression) and on the lactose, since it reduces its uptake (inducer exclusion).
What for?(AKA Applications)
We figured that such a system could be useful for two main purposes.
1. Controlled Gene Expression Trigger
Manipulating gene expression using most of the current protocols in molecular biology usually relay either on a strong overexpression of a protein or on its total silencing. Once induced, both these two extreme perturbations are generally unmodifiable (so that this approaches, although shedding light on the general role of the candidate gene, are rough indicators of the effect of its real physiological transcriptional level). Our genetic device can limit these drawbacks offering 1) a controlled on-off transition of the protein expression at will (by dosing the extracellular IPTG input) and 2) its graded transcriptional level (playing with the extracellular Glucose concentration), representing a powerful tool for biologists. Moreover, in addition to the ability to control the timing and the extent of the induction, our device has the additional advantage to guarantee that small changes in the extracellular concentration of the inducer IPTG would not affect the stability of the level of the expression.
2. Glucose Sensor
The second application takes advantage of the external glucose dependence. In fact, we know that the expression of the system is stable, but its intensity depends on the glucose. We can reverse the perspective and observe the intensity of the expression to infer the abundance of glucose in the medium.
Figure 5. Schematic view of our device
Mathematical Model
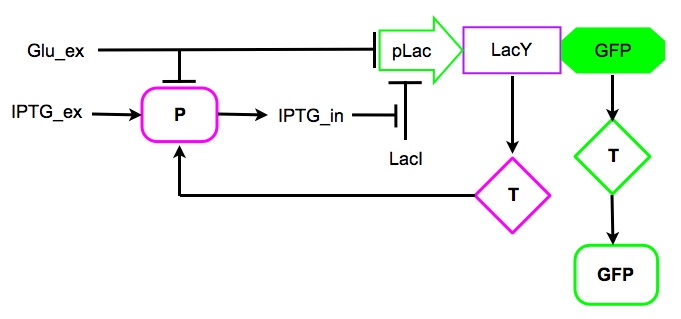
Based on the our knowledge on the [http://en.wikipedia.org/wiki/Lac_operon Lac operon] and on the works of Santilliàn et al. (2004, 2007), we decided to explore the behavior of a possible synthetic circuit which mimics the genetic circuit by modeling the parts depicted in Figure 6.
Figure 6. The core of the synthetic circuit as modelled in the subsequent equations. P and T represent the Protein and the Transcript of the corresponding genes.
Model Equations
Let’s start with the state model equations. They represent the variation over time (derivative d/dt) of the intracellular level (in molecules per bacterium, mpb) of:
- 1. the transcript of lacY (T);
- 2. the membrane permease coded by LacY (P)
- 3. IPTG: this is an artificial Lactose equivalent that is un-metabolizable by the cell. This allows us to neglect the effects of the other genomic Lac Operon Genes.
These equations are sufficient to model the dynamics relevant to the functioning of the system and nor overly complex to avoid biologist dejection.
The inputs of this system are the extracellular concentrations of Glucose and IPTG, that are processed by the non linear functions described in the table below.
| | Glucose Dependent LacY Transcription Inhibitor | Plot |
|---|---|---|
| | IPTG Dependent LacY Transcription Enhancer | Plot |
| | Glucose Dependent IPTG Uptake Inhibitor | Plot |
| | IPTG Dependent IPTG Uptake Enhancer | Plot |
and have their own formulas and trends.
Since our output is the reporter protein GFP we need another equation to link the protein LacY (P) to GFP: in first approximation, we simply assume:
All the genomic parameters (from Santillàn et al, 2007) are defined here, while the parameters adopted to model the synthetic parts are in the are defined in the table of Synthetic Parameters.
Numerical Simulation
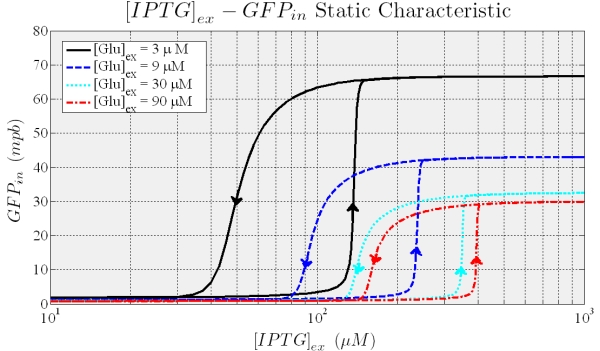
To analyse the circuit’s behaviour under different stimulations we implement model equations in simulink (Mathworks). As model input we impose a periodic change in the extracellular IPTG levels (slow enough to assume that the system is near to the equilibrium for each IPTG value) while Glucose is kept constant. Several simulations were ran by assigning a different value to Glucose. The results are shown in Figure 7: the system shows hysteresis, distinct thresholds depending on the Glucose and, once induced, constant level of expression. Notable, the circuit shows bistability (two stable equilibria) for IPTG values between the thresholds.
Figure 7. Shows the typical hysteresis profile, different curves are due to different values of the external Glucose.
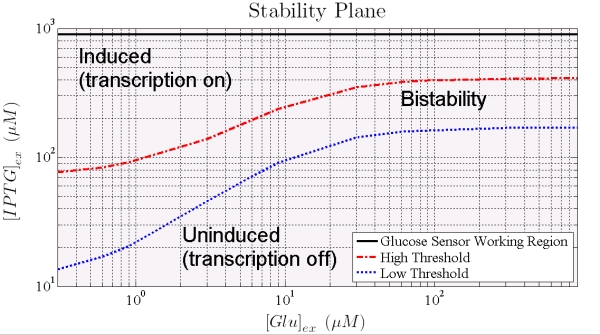
Dependence of IPTG thresholds on Glucose are shown in Figure 8: the points that lie between the thresolds are values (of the inputs) at which the system exhibit bistability; that is, we are not able of predict the final state with no information on the initial state. Outside these values, system reaches without any doubts, steady induced or uninduced state.
Figure 8. Shows how the 2 thresholds values change for varying levels of external glucose. All the points that lie between the two curves represent states for which the trascription can occur or not, the points below the blue curve corrspond pLac full represion (trascription off) while points over the red curve correpond to condition with Plac partially reppressed (trascription on).
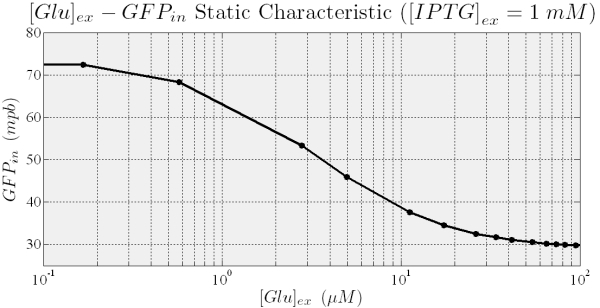
When the higher threshold is crossed the trascripion takes places with an expression level unsesitive to ITPG stimulus (see Figure 7). In this condition (i.e. IPTG 900 microM), the saturation level depends on the Glucose (see figure 8), and the circuit acts like a glucose sensor.
Figure 9.Shows that the final level of GFP concentration (output of the system) in the fully induced steady state depends on the External Glucose.
In conclusion, numerical simulation predictes a circuit functionality which is coherent with our initial speculation, i.e. the circuit presented in Figure 6 can operate like a Schmitt Trigger.
The values of few parameters were assigned with some uncertainty. In particular, we used a value of LacI that is about 30 times the genomic one for a circuit copy number of 10. Since these values can affect the model prediction we will perform specific experiment to identify this paramiters. However, the LacI values was incresed in order to have trigger off for low vales of IPTG. To realize this increase we decided to decide to build a sub-circuit where the LacI gene is under the control of pTET promotor.
At the same way, the GFP steady state expression decreases with extracellular Glucose (see Figure 9), while we want, for an intuitive use of the sensor a fluorescent signal that increases with Glucose. To obtain this behavior we plan to build a sub-circuit that acts like an inverter, with an RFP under the control of pλ, and to put in the main circuit the cI gene which encode for the protein that regulates pλ.
Image Acquisition and Analysis
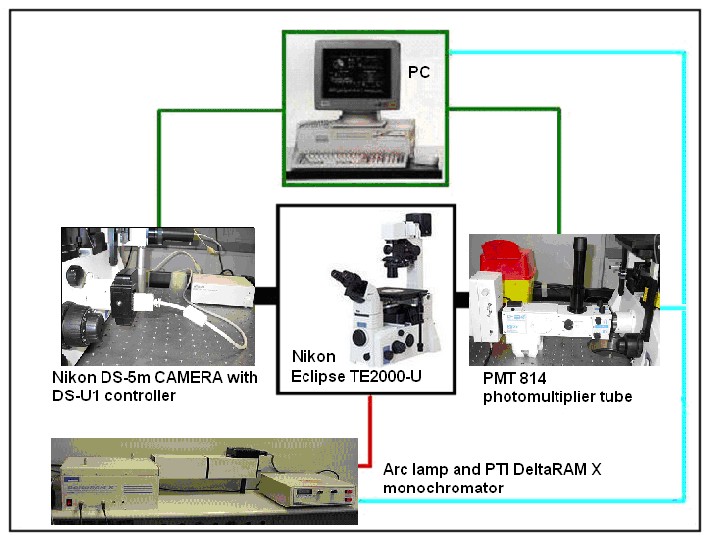
Image Acquisition System
Figure 10. Image acquisition system based on the fluorescence microscope.
For complete description of Acquisition System, see here.
Image Acquisition
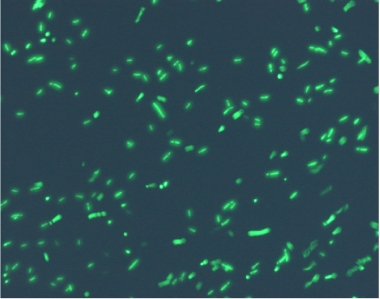
Using the experimental set up, we obtain short movies of fluorescent bacteria; then we extract several frames from each of them, using a dedicated software. Example images are shown below.
Figure 11.Fluorescent bacteria
Image Elaboration
Since we need to know how much of the total image area is occupied by bacteria, we process the images with a segmentation algorithm implemented in Matlab.
Here are the processing phases:
- the RGB image is read and the green channel is extracted;
- a morphological top hat filtering is performed on the grayscale image;
- by means of an adaptive threshold, the image is tranformed in a binary one;
- the distance between every point and the nearest black pixel is calculated;
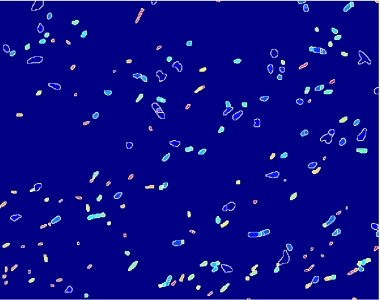
- on this last image the [http://en.wikipedia.org/wiki/Watershed_%28algorithm%29 watershed] algorithm is applied: every pixel is assigned a label, depending on the segmentated region it belongs; then, every labeled region is represented with a different colour, as in the images shown below;
Figure 12.Algorithm output.
- the area of every segmented region is calculated, checking that the summation of these areas (including the background) balances the image total area;
- two complementary matrices are created:
1) ImageWithOutBackground, containing the intensity positive values corresponding only to pixels recognised as bacteria, with zeros elsewhere;
2) ImageBackground, which contains the intensity positive values of pixels recognised as background, with zeros as other entries;
- with a summation over all the ImageWithOutBackground matrix' entries, the total intensity is obtained;
- dividing this value by the total bacteria area, the output is the normalized intensity we use to compare the fluorescence of different kinds of bacteria.
This image acquisition and elaboration protocol has been validated with a series of measures. For the results, see here.
Thanks to Camilo Melani for his competence and kindness during the algorithm implementation.
Biodevice
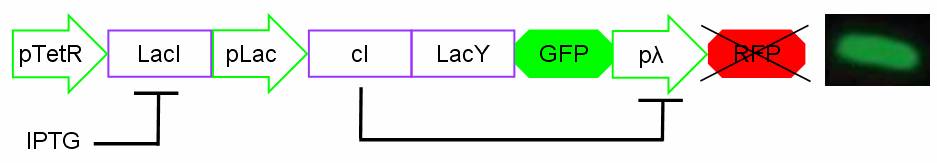
Components
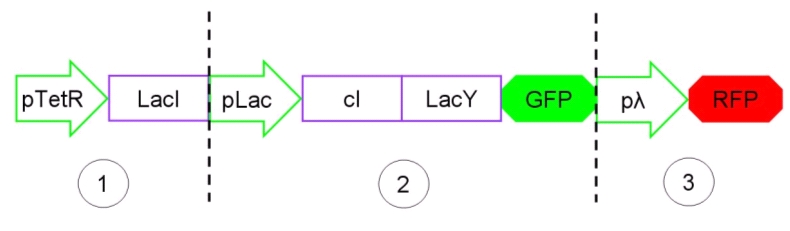
The Genetic Schmitt Trigger [http://partsregistry.org/Part:BBa_I763029 (I763029)], built up with iGEM 2007 Biobricks, consists of 3 main parts combined in the same plasmid: pTeTR-LacI [http://partsregistry.org/Part:BBa_I763026 (I763026)], pLac-cI-LacY-GFP [http://partsregistry.org/Part:BBa_I763019 (I763019)], pλ-RFP [http://partsregistry.org/Part:BBa_I763007 (I763007)].
Each part displays a specific function depending on the promoter and the coded gene(s).
Figure 13: The Genetic Schmitt Trigger biodevice ([http://partsregistry.org/Part:BBa_I763029 I763029]).
- 1.pTetR-LacI [http://partsregistry.org/Part:BBa_I763026 (I763026)] codes for LacI gene [http://partsregistry.org/Part:BBa_C0012 (C0012)] regulated by pTetR [http://partsregistry.org/Part:BBa_R0040 (R0040)] inverting promoter. pTetR can be considered a constitutive promoter in absence of tetracycline (or its analog aTc). Its action is inhibited by the addition of this antibiotic. This promoter regulates the expression of LacI gene whose protein inhibits the activation of pLac promoter. This part is important to make up for endogenous LacI and to prevent pLac activation in absence of induction with lactose or IPTG.
- 2.pLac-cI-LacY-GFP [http://partsregistry.org/Part:BBa_I763019 (I763019)]: LacY permease [http://partsregistry.org/Part:BBa_J22101 (J22101)] controlled by pLac promoter [http://partsregistry.org/Part:BBa_R0010 (R0010)] introduces a positive feedback necessary for hysteresis. LacY is a membrane transporter allowing the uptake of lactose (or IPTG) in the cell. Lactose (or IPTG) on his own, causes the LacI repressor release from pLac operators. At the same time GFP proteins [http://partsregistry.org/Part:BBa_J04031 (J04031)] are produced as reporters of the induction. We also introduced the gene for cI repressor [http://partsregistry.org/Part:BBa_C0051 (C0051)] that binds to the cI regulator [http://partsregistry.org/Part:BBa_R0051 (R0051)] and inhibits its action.
- 3.pλ-RFP [http://partsregistry.org/Part:BBa_I763007 (I763007)] acts as reporter of standard condition. Pλ promoter [http://partsregistry.org/Part:BBa_R0051 (R0051)] is a constitutive promoter from phage-λ. Here it regulates RFP protein [http://partsregistry.org/Part:BBa_E1010 (E1010)] expression and it is inhibited by cI repressor [http://partsregistry.org/Part:BBa_C0051 (C0051)]. So, when external glucose is high, pLac transcription level, cI and GFP production are high, while RFP production is low. When external glucose is low pLac transcription level, cI and GFP production are low while RFP production is high. As a consequence, we can consider RFP fluorescence levels as an indicator of glucose concentration in culture medium.
How it works
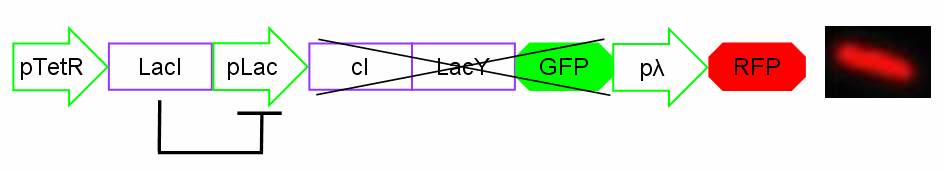
In standard conditions, without IPTG induction pLac promoter is repressed by the LacI repressor binding on the operator sites and cI, LacY and GFP proteins are not expressed. In this condition pλ promoter is active and RFP is expressed. The cells show red fluorescence.
Figure 14: Project in action: no IPTG in the medium.
For induction IPTG is added in the culture medium. Due to inductor binding to LacI repressor, Plac promoter is activated and cI, LacY and GFP genes are expressed. In this condition pλ promoter is repressed by cI inhibitor and so RFP is not expressed. The cells show green fluorescence.
Figure 15: Project in action: IPTG in the medium.
Intermediates
The table below shows all the intermediates that we have obtained to build up our final device. For each part you can find:
-in the first column the link to the Registry;
-in the second column the components for each part;
-in the third column the plasmid the part has been cloned in;
-in the fourth the insert length after double digestion with Xba1/Pst1 enzymes linked to the electrophoresis gel run;
-in the fifth column cell vitality after transformation;
-in the sixth column fluo data for each part.
| Igem Code | Fragment | Plasmid | Length (bp) | Vitality | Fluo | |
|
|
|
|
|
Clicking on the length of the fragment you will be shown the electrophoresis gel of the digestion of the fragment excised from the plasmid by cutting it with a double digestion using Xba and Pst1 enzymes.
Biodevice Characterization
Fluorescence intensity dependence on Promoter type
Some devices ([http://partsregistry.org/Part:BBa_I763004 I763004], [http://partsregistry.org/Part:BBa_I763035 I763035] and [http://partsregistry.org/Part:BBa_I763011 I763011]) have been characterized in order to gain useful information to identify the model. We chose to characterize the behavior of devices without feedback.
Three different promoters have been observed to stabilish the transcription efficiency.
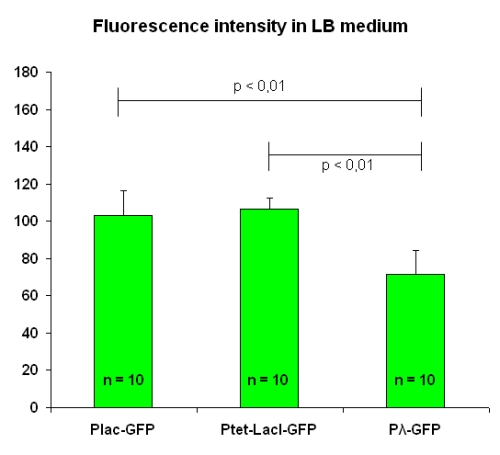
All of them grew into LB medium. The experimental setup conditions are the same for each test, and the OD value was mantained at 1.2. We took pictures of two fields from each of the five slides we prepared; then, we calculated the mean value for each slide and finally the mean and the standard deviation on these values. Results are in figure below:
Fluorescence intensity levels for [http://partsregistry.org/Part:BBa_I763004 I763004] and [http://partsregistry.org/Part:BBa_I763035 I763035] are comparable, while [http://partsregistry.org/Part:BBa_I763011 I763011] beams a lesser garde of fluorescence.
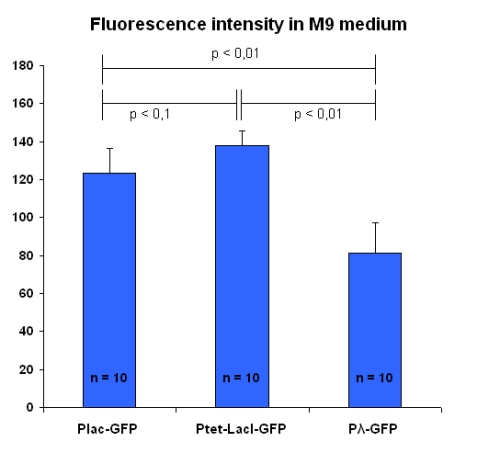
These tests have been repeated, in the same experimental conditions, with bacteria into M9 solution; results are in figure below:
In this case, the three promoters produce levels of fluorescence fairly different.
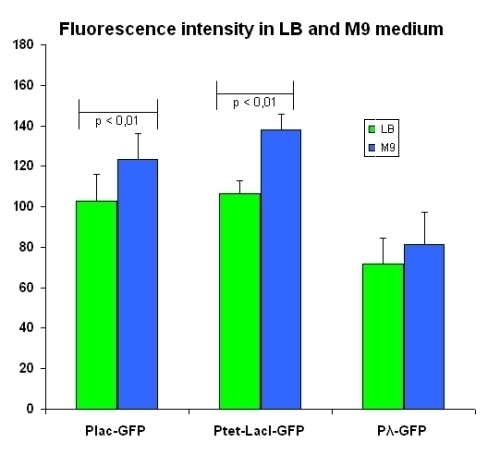
Comparison between fluorescence elicited by two distinct Mediums
The output data have been re-elaborated to highlight the difference in fluorescence intensity between the same devices into two different media, LB and M9. The comparison is shown in figure below:
Complete data set, summarized in the table below, are here.
| LB | M9 | ||
|---|---|---|---|
| I763004 | pLac-GFP | 102.9 ± 13.4 | 123.2 ± 13.1 |
| I763035 | pTetR-LacI-GFP | 106.6 ± 6.0 | 138.1 ± 7.7 |
| I763011 | pλ-GFP | 71.6 ± 12.3 | 81.3 ± 13.9 |
Acknowledgments
Our Team was funded by:
- European Union SYNBIOCOMM project

- Ser.In.Ar. Cesena
- University of Bologna - Cesena Campus
Overview Table
| Our Team | Project Design | Project Results |
|
|