Bologna
From 2007.igem.org
"Ὁ βίος βραχὺς, ἡ δὲ τέχνη μακρὴ, ὁ δὲ καιρὸς ὀξὺς, ἡ δὲ πεῖρα σφαλερὴ, ἡ δὲ κρίσις χαλεπή"
"Vita brevis, ars longa, occasio praeceps, experimentum periculosum, iudicium difficile"
"Art is long, life is short, opportunity is fleeting, experience is deceitful, judgement is difficult"
"La vita è breve, l'arte è lunga, l'occasione fuggevole, l'esperimento pericoloso, il giudizio difficile"
(Aforisma di Ippocrate di Coo)
Contents |
About Us
Welcome to Bologna’s IGEM Wiki!
Our team consists of Francesco Pasqualini, Guido Costa, Iros Barozzi, Silvia Tamarri, Francesca Buganè, and Michela Mirri, all soon-to-be juniors majoring in Biomedical Engineering at Bologna University; and Alice Pasini, a just graduated student.
We are advised by [http://www.ing2.unibo.it/Ingegneria+Cesena/Strumenti+del+Portale/Cerca/paginaWebDocente.htm?NRMODE=Published&TabControl1=TabContatti&UPN=emanuele.giordano%40unibo.it Dr Emanuele Giordano], Lecturer in Biochemistry and [http://www-micro.deis.unibo.it/cgi-bin/user?tartagni Prof. Marco Tartagni], Professor of Electronics. We are grateful to our advisors for their time and support!
Our instructors are [http://www.ing2.unibo.it/Ingegneria%20Cesena/Strumenti%20del%20Portale/Cerca/paginaWebDocente?UPN=silvio.cavalcanti@unibo.it Prof. Silvio Cavalcanti], Professor of Biomedical Engineering, Francesca Ceroni, Graduate student in Pharmaceutical Biotechnologies, Sara Montagna, Graduate student in Biomedical Engineering and [http://www-micrel.deis.unibo.it/~christine/ Christine Nardini], PhD in Bioengineering.
Our Project
Introduction
Our goal is the realization of a genetic circuit able to implement the functionality typical of an electronic device called Schmitt Trigger(as defined by its inventor [http://www.otto-schmitt.org/ Dr. Otto H. Schmitt]).
Pardon me, what do you mean? (AKA Functional Requirements)
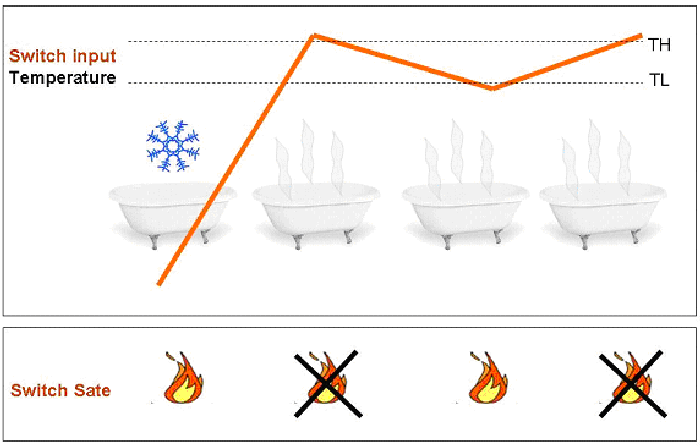
The main characteristics of this device is to be a “smart” switch. A switch is a device that, given a change in some environmental condition causes an appropriate modification in the output, for instance the output changes from on to off. Often the environmental change is the modification of the value in some parameter (input of the switch) that describes the environment. The device switches for a given value (threshold) of the input. So far so good, however, if the switch input has a value that continually even minimally changes across the threshold, the system will keep going on and off, wasting energy and leaving the system in an unstable state. To avoid all this we need a “smart” switch. Basically, this device switches on or off at two different threshold (High and Low threshold, TH and TL respectively) depending on the history of the system. So, according to the state of the system, the threshold for switching will change. This can solve the instability problem, since the variations that cause instability now must be as high as the difference between the two thresholds (see Figure 1).
Figure1 Once the temperature passes the lower threshold, for small changes of the temperature (between TL and TH) the system is able to automatically modify its state (on-off), so that the average temperature of the water remains stable (and so does your mood if you are in the bath tub).
The reasons why the “smart” switch works that well are due to the fact that (1) the system it constitutes has two stable states, that the system reaches when below the lower threshold (TL) or above the higher threshold (TH); and (2) the system has a positive feedback. All this allows for hysteresis (qui ci va il link a quello che segue sotto) to take place, this is the fundamental property we want to reproduce in our genetic circuit.
How? (AKA Technical Requirements)
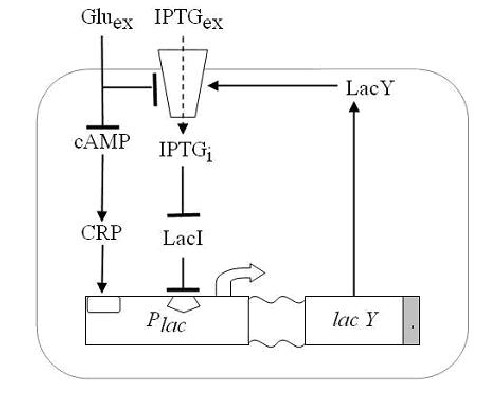
We wanted to reproduce the same principle with a genetic circuit. To do so we exploited one of such system, existing in the complex of genes that form the Lac Operon shown in Figure 4. Namely, E.coli can either survive by metabolizing glucose or, in case glucose is missing, lactose. Lactose or glucose metabolism-modes are the two stable state of our system. To perform experiments we use IPTG a structural analog of lactose that cannot be metabolized. Thus the input of our system are the external concentrations of Glucose and IPTG (Gluex and IPTGex respectively). Since lactose metabolism is more energy consuming, usually all the apparatus that takes care of the lactose metabolism is repressed. That is [http://en.wikipedia.org/wiki/Lac_operon the promoter pLac ] is constitutively repressed thank to the presence of the LacI protein that inhibits the transcription of the genes downstream of the operon ([http://bcs.whfreeman.com/mga2e/pages/bcs-main.asp?s=132&n=003&i=283&v=&o=&ns=0&uid=0&rau=0 this] is how it works). When there is IPTG in the environment of the cell, several concurrent processes take place. IPTG enters through the membrane and after some processing it is able to remove the repressor LacI, thus allowing the transcription of genes lacY that in turn enhance the IPTG uptake and increase its action on the repressor LacI. This represents the positive feedback loop we need.
Figure 2 Schematic overview of the Lac operon. As it is the system can already perform like a Schmitt trigger, for certain values of the inputs. However there is another mechanism that allows the fine tuning of the system: glucose has a double action both on a molecule called cAMP that it bounds to, impeding its action on pLac expression (catabolite repression) and on the lactose, since it reduces its uptake (inducer exclusion).
What for?(AKA Applications)
We figured that such a system could be useful for two main purposes.
Controlled Gene Expression Trigger. To control the expression of a gene, current systems require the delivery of large amount of inducers, to make sure the transcription of the gene of interest takes place. Such quantities of inducer are often non physiologic and can perturb the whole system in uncontrollable ways. With our genetic device we could control the amount of the inducer to be produced. The inducer in fact could represent the output of our device. Besides being able to control the quantity of inducer, our device has the additional advantage to guarantee that small changes in the promoter that forces the inducer’s production (the input of our device) would not change the inducer’s quantity, thank to the robustness of the threshold.
Glucose Sensor. The second application takes advantage of the external glucose dependence. In fact, we know that the expression of the system is stable, but its intensity depends on the glucose. We can reverse the perspective and observe the intensity of the expression to infer the abundance of glucose.
Figure 3. Schematic view of our device
Acknowledgments
Our Team was funded by
- European Union SYNBIOCOMM project

- Ser.In.Ar. Cesena
- University of Bologna - Cesena Campus
Overview Table
| Our Team | Project Design | Project Results |
|
|