Imperial/Wet Lab/Lab Notebook/2007-08-29
From 2007.igem.org
(→Construction of pT7-GFP) |
m (→Construction of pT7-GFP) |
||
| (4 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Template:IC07navmenu}} | {{Template:IC07navmenu}} | ||
<html><div id="maincol"></html> | <html><div id="maincol"></html> | ||
| - | + | __NOTOC__ | |
= 29 August 2007 = | = 29 August 2007 = | ||
| Line 9: | Line 9: | ||
This is to test if the commmercial cell extract and pre-incubation mix, if mixed in a ratio of 1:1 with the home made cell extract and reaction buffer, will produce sufficiently satisfying results to be used for carrying out the rest of the experiments for CELL BY DATE and INFECTOR DETECTOR. | This is to test if the commmercial cell extract and pre-incubation mix, if mixed in a ratio of 1:1 with the home made cell extract and reaction buffer, will produce sufficiently satisfying results to be used for carrying out the rest of the experiments for CELL BY DATE and INFECTOR DETECTOR. | ||
<br><br> | <br><br> | ||
| - | Protocol can be found here under [ | + | Protocol can be found here under [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Experimental_Design/Phase1/Protocol_3.2 Phase 1-In vitro testing] on the experimental design page. |
<br><br> | <br><br> | ||
| - | |||
It was observed that fluorescense did not increase after 4 hours incubation. | It was observed that fluorescense did not increase after 4 hours incubation. | ||
| Line 26: | Line 25: | ||
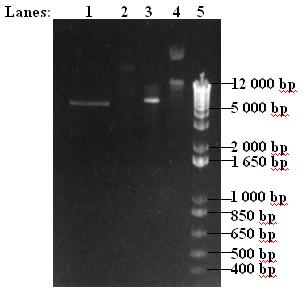
[[Image:IC07_T7GEL.JPG|200px]] | [[Image:IC07_T7GEL.JPG|200px]] | ||
| - | Protocols can be found at [ | + | Protocols can be found at [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Notebook/General_Protocols#S30.2FS12_Cell_Extract#Electrophoresis Electrophoresis] in the general protocols page |
#Re-digested 15 μl of insert (thrice the previous amount) | #Re-digested 15 μl of insert (thrice the previous amount) | ||
| Line 34: | Line 33: | ||
'''Formation of Vesicles''' | '''Formation of Vesicles''' | ||
| - | With the suspensions prepared [[ | + | With the suspensions prepared [[Imperial/Wet Lab/Lab Notebook/2007-08-28|the day before]], the following steps were taken: |
* 2ml of suspension was taken to prepare an interface according to protocol. | * 2ml of suspension was taken to prepare an interface according to protocol. | ||
* 25μl of 100x diluted GFP solution was used to prepare the emulsion; stirred gently with magnetic stirrer. | * 25μl of 100x diluted GFP solution was used to prepare the emulsion; stirred gently with magnetic stirrer. | ||
Latest revision as of 21:07, 26 October 2007

29 August 2007
Investigate the efficiency of a 1:1 mixture of commercial and homemade extract with pTet at 37oC
This is to test if the commmercial cell extract and pre-incubation mix, if mixed in a ratio of 1:1 with the home made cell extract and reaction buffer, will produce sufficiently satisfying results to be used for carrying out the rest of the experiments for CELL BY DATE and INFECTOR DETECTOR.
Protocol can be found here under [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Experimental_Design/Phase1/Protocol_3.2 Phase 1-In vitro testing] on the experimental design page.
It was observed that fluorescense did not increase after 4 hours incubation.
Construction of pT7-GFP
- Insert and vector digests were run in a 1% agarose gel
- 1. 20 μl insert digest
- 2. 10 μl uncut insert
- 3. 3 μl vector digest
- 4. 3 μl uncut vector
- 5. 1 kb DNA ladder
- Insert band was too faint to be cut out
Protocols can be found at [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Notebook/General_Protocols#S30.2FS12_Cell_Extract#Electrophoresis Electrophoresis] in the general protocols page
- Re-digested 15 μl of insert (thrice the previous amount)
Vesicles
Formation of Vesicles
With the suspensions prepared the day before, the following steps were taken:
- 2ml of suspension was taken to prepare an interface according to protocol.
- 25μl of 100x diluted GFP solution was used to prepare the emulsion; stirred gently with magnetic stirrer.
5 samples prepared:
- Sample 1: 1ml of 50ml POPC/dodecane/GFP emulsion added over interface prepared with 2ml of POPC/dodecane suspension.
- Sample 2: 1ml of 50ml DOPC/Span-80/Mineral Oil/GFP emulsion added over interface prepared with 2ml of DOPC/dodecane suspension.
- Sample 3: 2ml of POPC/dodecane/GFP emulsion, with no previously prepared interface.
- Sample 4: 2ml of 40ml DOPC/Span-80/Mineral Oil/GFP emulsion, with no previously prepared interface.
- Sample 5: 2ml of 10ml DOPC/Span-80/Mineral Oil/GFP emulsion, with no previously prepared interface.
Samples 1 and 2 were centrifuged at 120x g. Samples 3, 4, and were not centrifuged.
All samples were left overnight.
Results
No samples were observed today.
Preparations
No preparations were made today.