Imperial/Wet Lab/Lab Notebook/2007-09-03
From 2007.igem.org
(→3 September 2007) |
m (→Construction of pT7-GFP) |
||
| (3 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Template:IC07navmenu}} | {{Template:IC07navmenu}} | ||
<html><div id="maincol"></html> | <html><div id="maincol"></html> | ||
| - | + | __NOTOC__ | |
= 3 September 2007 = | = 3 September 2007 = | ||
| Line 8: | Line 8: | ||
#Minipreped the 5 overnight cultures | #Minipreped the 5 overnight cultures | ||
| - | Protocols can be found at [ | + | Protocols can be found at [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Notebook/General_Protocols#S30.2FS12_Cell_Extract#Miniprep| Miniprep] in the general protocols page |
#Digested 5μl of DNA with Nde1 and BamH1 | #Digested 5μl of DNA with Nde1 and BamH1 | ||
| Line 24: | Line 24: | ||
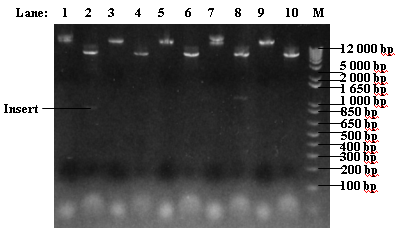
#Colonies 1 and 4 show the correct-size insert upon digestion | #Colonies 1 and 4 show the correct-size insert upon digestion | ||
| - | [[Image: | + | [[Image:ICGEMS T7GEL4.PNG|350px]] |
| - | Protocols can be found at [ | + | Protocols can be found at [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Notebook/General_Protocols#Electroporation| Electroporation] in the general protocols page |
#Transformed recombinant plasmid DNA into BL21 | #Transformed recombinant plasmid DNA into BL21 | ||
| Line 32: | Line 32: | ||
#Left plates at 37°C overnight | #Left plates at 37°C overnight | ||
| - | Protocols can be found at [ | + | Protocols can be found at [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Notebook/General_Protocols#S30.2FS12_Cell_Extract#Transformation| Transformation] in the general protocols page |
==Maxiprep of Biobricks== | ==Maxiprep of Biobricks== | ||
| Line 42: | Line 42: | ||
</font> | </font> | ||
| - | Protocols can be found at [ | + | Protocols can be found at [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Notebook/General_Protocols#S30.2FS12_Cell_Extract#Maxiprep| Maxiprep] in the general protocols page |
[[Image:ICGEMS Gel 3 9.png]] | [[Image:ICGEMS Gel 3 9.png]] | ||
Latest revision as of 14:45, 26 October 2007

3 September 2007
Construction of pT7-GFP
- Minipreped the 5 overnight cultures
Protocols can be found at [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Notebook/General_Protocols#S30.2FS12_Cell_Extract#Miniprep| Miniprep] in the general protocols page
- Digested 5μl of DNA with Nde1 and BamH1
- Checked the digestion on 1% agarose gel
- 1. Colony 1
- 2. Colony 1 digested
- 3. Colony 2
- 4. Colony 2 digested
- 5. Colony 3
- 6. Colony 3 digested
- 7. Colony 4
- 8. Colony 4 digested
- 9. Colony 5
- 10. Colony 5 digested
- Colonies 1 and 4 show the correct-size insert upon digestion
Protocols can be found at [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Notebook/General_Protocols#Electroporation| Electroporation] in the general protocols page
- Transformed recombinant plasmid DNA into BL21
- Plated transformed cells onto LB + Kan plates
- Left plates at 37°C overnight
Protocols can be found at [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Notebook/General_Protocols#S30.2FS12_Cell_Extract#Transformation| Transformation] in the general protocols page
Maxiprep of Biobricks
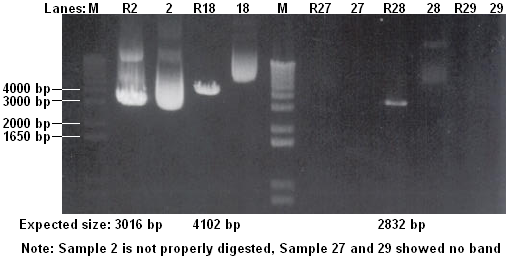
- Maxipreped 2 Biobricks
- 2. BBa_I13422 [ptet-GFP]
- 18. BBa_T9002 [plux-GFP]
Protocols can be found at [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Notebook/General_Protocols#S30.2FS12_Cell_Extract#Maxiprep| Maxiprep] in the general protocols page
Conclusions:
- Part 2 is very concentrated!
- Part 27 and 29 are not cloned properly
Vesicles
Formation of Vesicles
For both the POPC/dodecane 10ml and DOPC/dodecane 10ml suspensions prepared today - without overnight incubation - the following steps were taken:
- 2ml of suspension was taken to prepare an interface according to protocol.
- 25μl of 100x diluted GFP solution was used to prepare the emulsion; stirred gently with magnetic stirrer.
NOTE: There was a mistake in labelling the containers for the two suspensions - both were mistakenly labelled as DOPC. When the mistake was spotted, it was impossible to distinguish between the POPC and DOPC containers. The labels used then were a best guess, and have been carried through the remainder of these results. So, please bear in mind that the results given could either be POPC or DOPC.
4 samples prepared:
- Sample 1: 1ml of POPC/dodecane/GFP emulsion added over interface prepared with 2ml of POPC/dodecane suspension.
- Sample 2: 1ml of DOPC/dodecane/GFP emulsion added over interface prepared with 2ml of DOPC/dodecane suspension.
- Sample 3: 2ml of POPC/dodecane/GFP emulsion, with no previously prepared interface.
- Sample 4: 2ml of DOPC/dodecane/GFP emulsion, with no previously prepared interface.
Samples 1 and 2 were centrifuged at 120x g. Samples 3 and 4 have been left overnight for sedimentation to occur.
Results
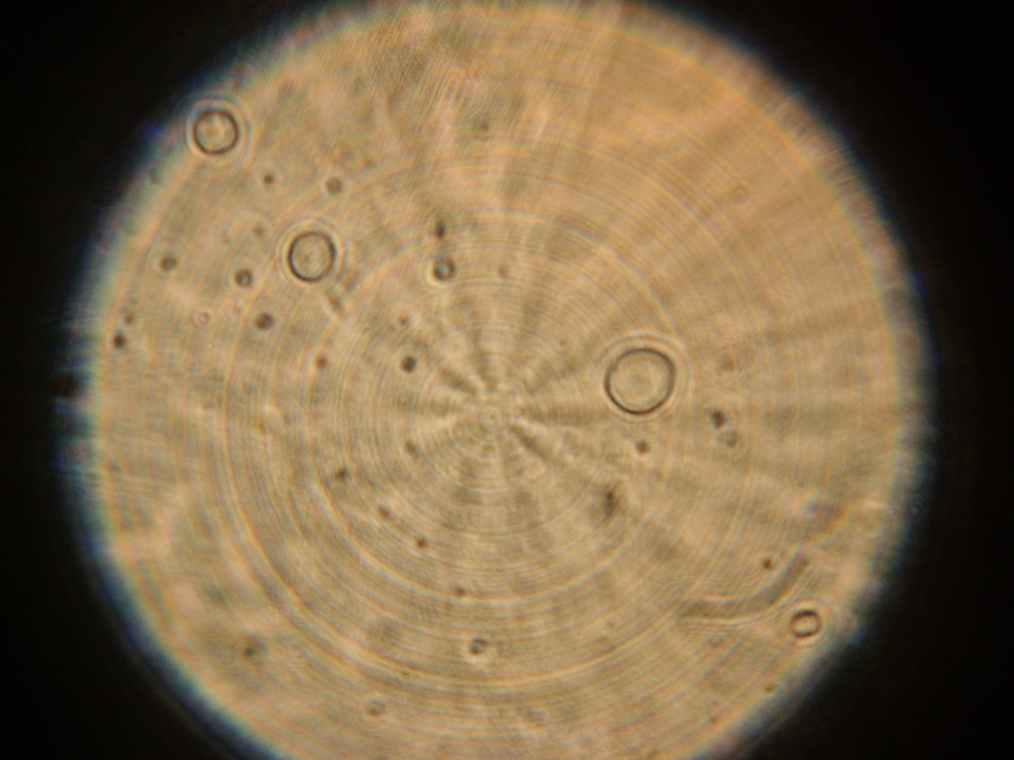
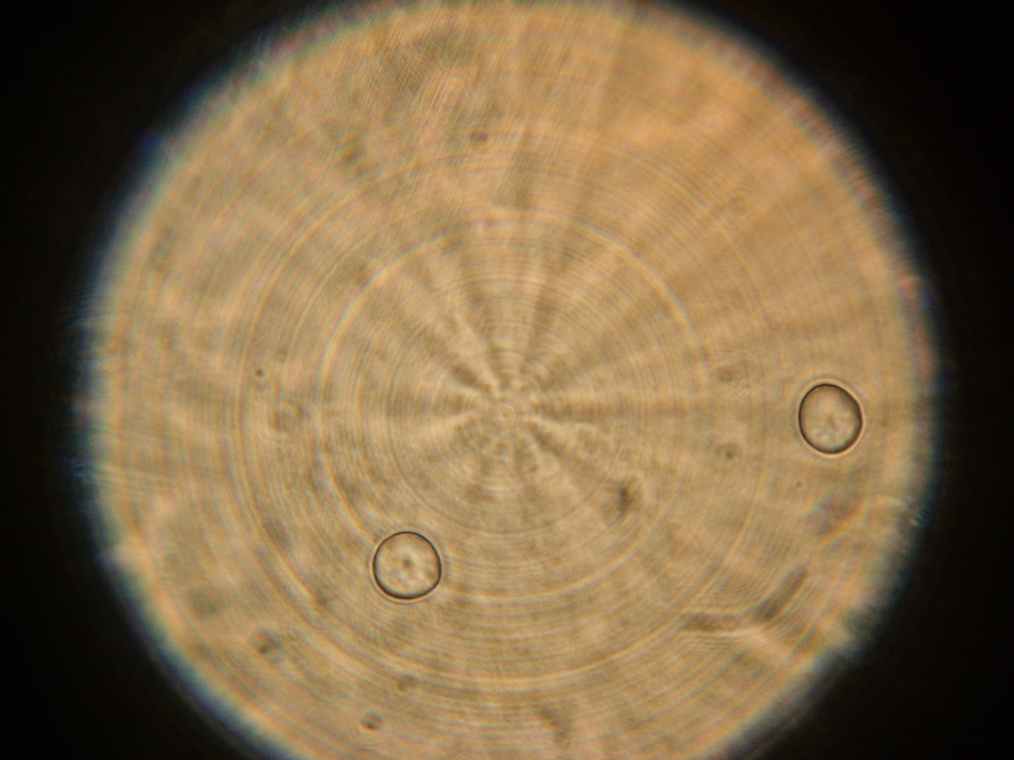
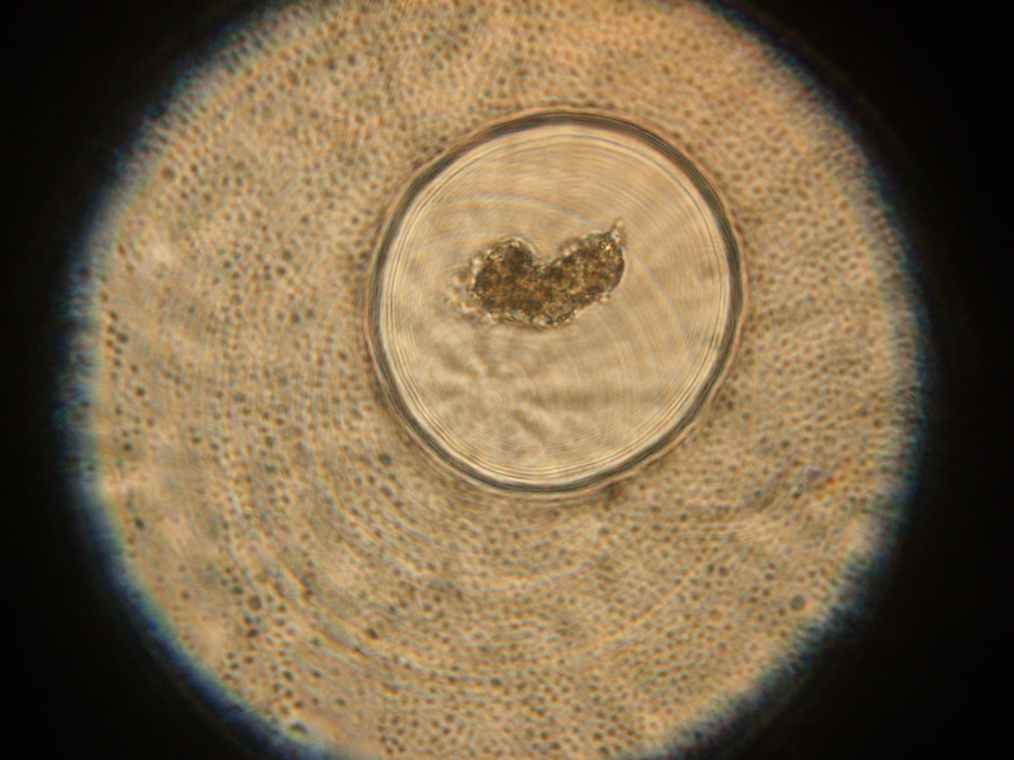
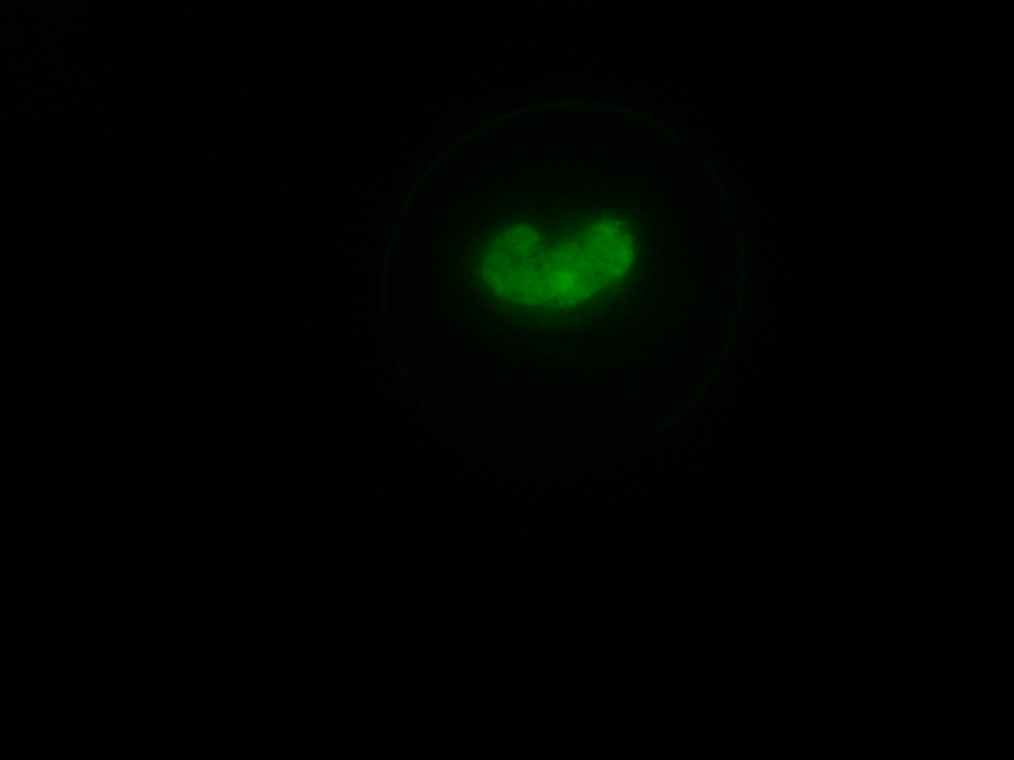
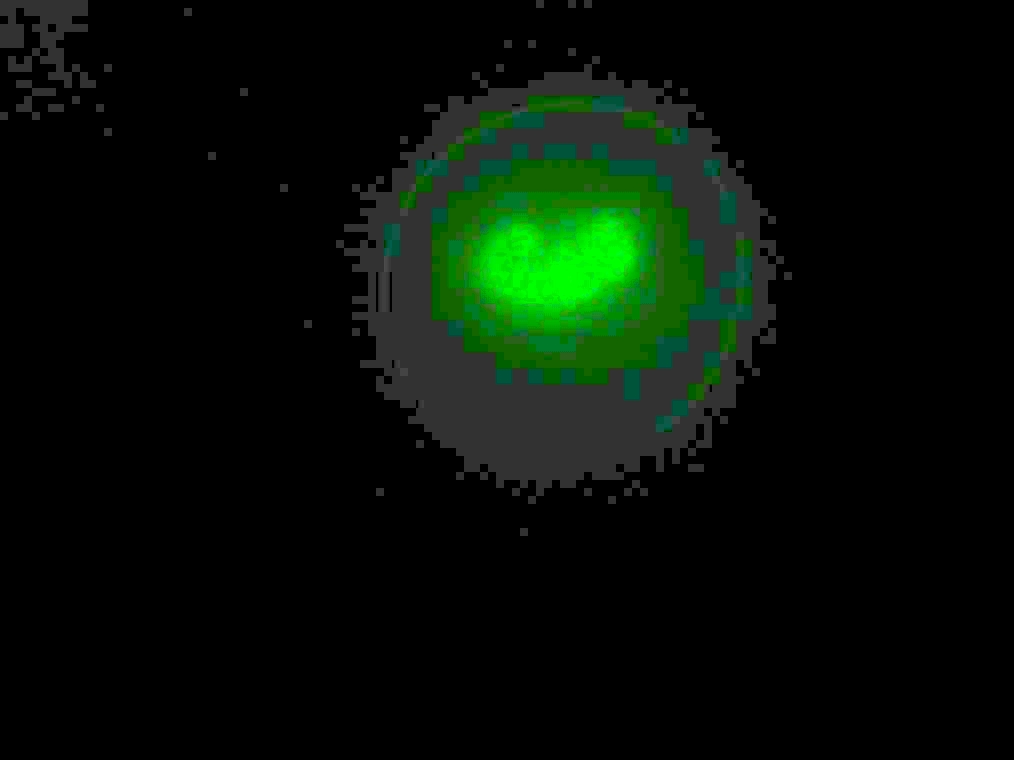
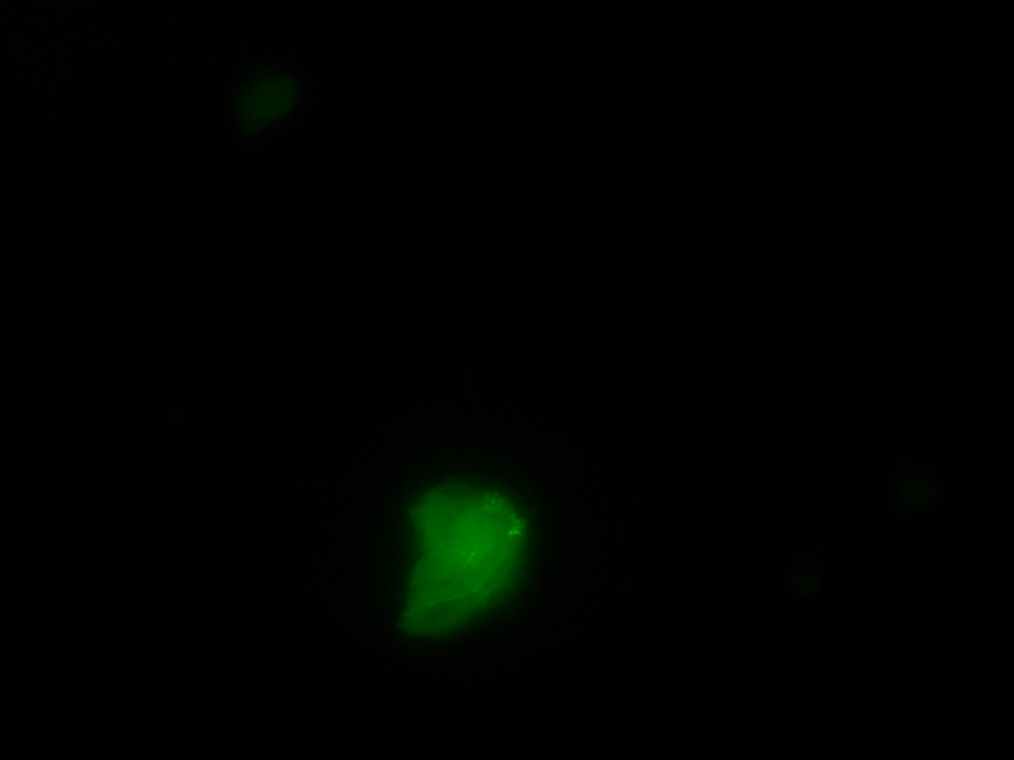
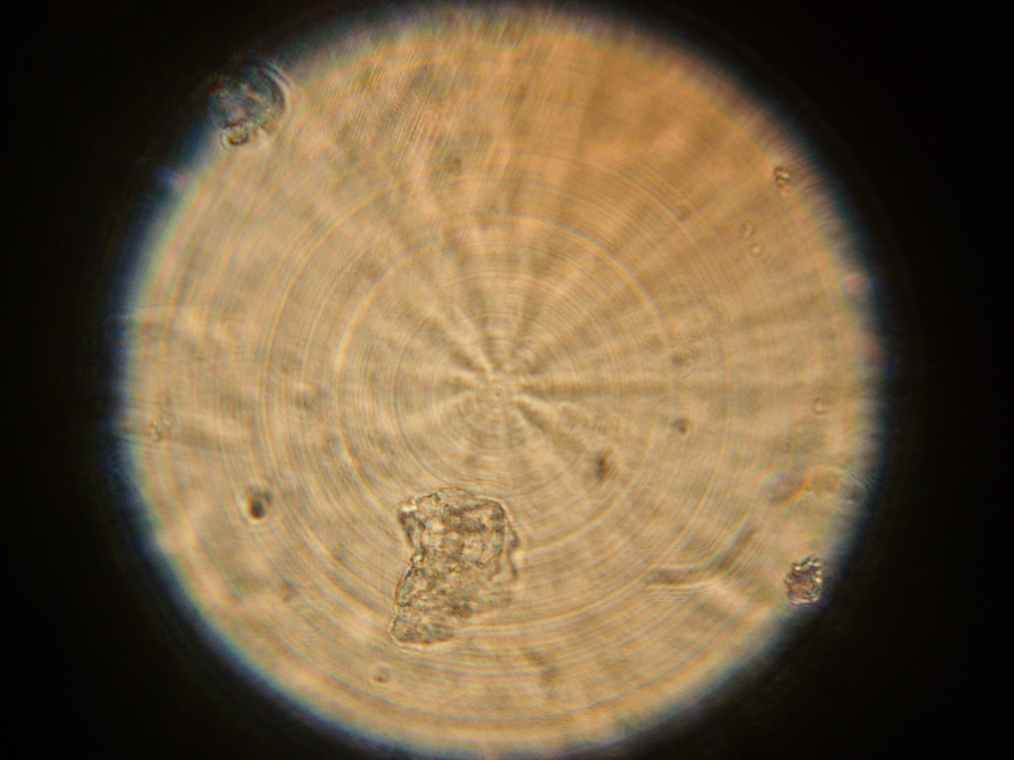
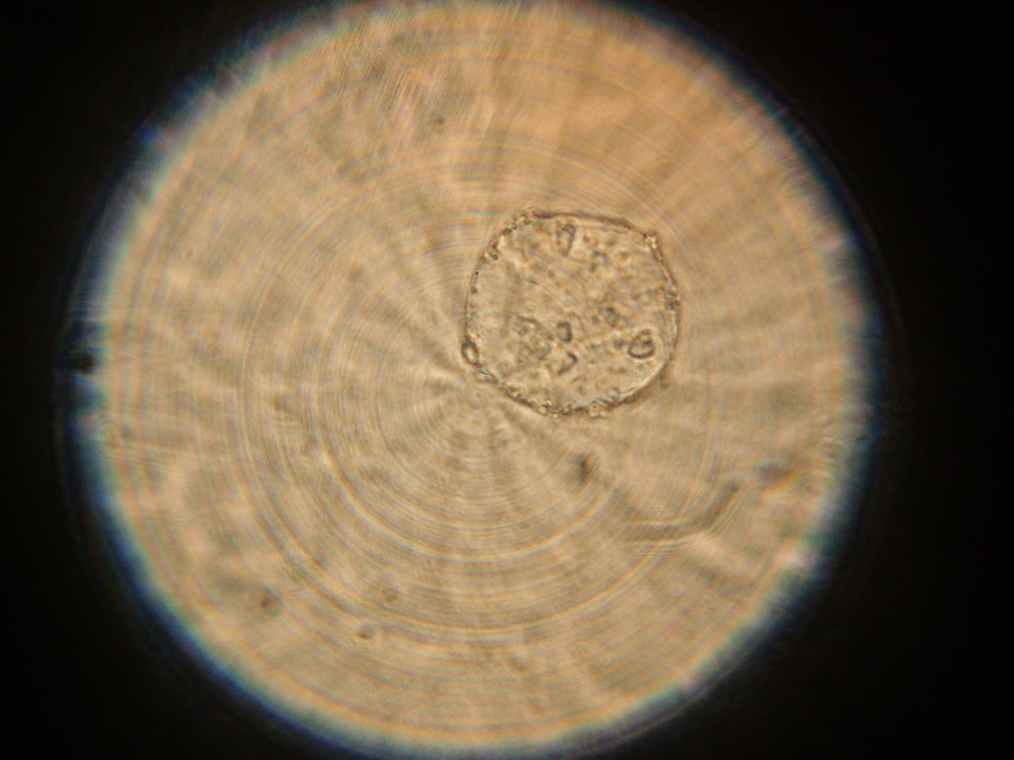
Samples 1 and 2 were collected for observation under the microscope. The following results were obtained:
- Sample 1: Vesicles observed, but not photographed.
- Sample 2: Vesicles observed.
Preparations
Three experiments were set up for tomorrow:
- One 100ml beaker with 250μl of POPC and 50ml of dodecane
- One 50mm by 25mm cylinder with 50μl of POPC and 10ml of dodecane
- One 50mm by 25mm cylinder with 50μl of DOPC and 10ml of dodecane
All desiccated and sonicated, and left on the bench overnight (not the incubator).