Imperial/Wet Lab/Lab Notebook/2007-08-24
From 2007.igem.org
m |
m (→In Vitro Testing of pTet 25<sup>o</sup>C using Home-Made Cell Extract with Commercial Pre-Incubation Mix) |
||
| (2 intermediate revisions not shown) | |||
| Line 8: | Line 8: | ||
Tested for the working condition of the DNA construct pTet-LuxR-pLux-GFP. This experiment will carry on until fluorescence reaches that of the control. | Tested for the working condition of the DNA construct pTet-LuxR-pLux-GFP. This experiment will carry on until fluorescence reaches that of the control. | ||
<br><br> | <br><br> | ||
| - | Protocol can be found here under [ | + | Protocol can be found here under [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Experimental_Design/Phase1/Protocol_2.2 Phase 1-In vitro testing] on the experimental design page. |
<br><br> | <br><br> | ||
| - | Results can here under [ | + | Results can here under [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Experimental_Design/Phase1/Results_2.2 Results] on the experimental design page |
| - | + | ||
==Preparation of Reaction Buffer for S30 Cell Extract== | ==Preparation of Reaction Buffer for S30 Cell Extract== | ||
| Line 30: | Line 29: | ||
Tested for the viability of the home-made cell extract. | Tested for the viability of the home-made cell extract. | ||
<br><br> | <br><br> | ||
| - | Protocol followed was according to the Promega one, and can be found here under [ | + | Protocol followed was according to the Promega one, and can be found here under [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Experimental_Design/Phase1/Protocol_2.1 Phase 1-In vitro testing] on the experimental design page. |
<br><br> | <br><br> | ||
| - | Results: It was observed that no fluorescence was produced during the incubation period of over 4 hours. | + | Results: It was observed that no fluorescence was produced during the incubation period of over 4 hours. |
| - | + | ||
==In Vitro Testing of pTet 25<sup>o</sup>C using Home-Made Cell Extract and Reaction Buffer== | ==In Vitro Testing of pTet 25<sup>o</sup>C using Home-Made Cell Extract and Reaction Buffer== | ||
| Line 46: | Line 44: | ||
==Vesicles Formation with GFP== | ==Vesicles Formation with GFP== | ||
'''Formation of Vesicles''' | '''Formation of Vesicles''' | ||
| - | Using POPC/dodecane suspension from [[Imperial/Wet Lab/Lab Notebook/2007-08- | + | Using POPC/dodecane suspension from [[Imperial/Wet Lab/Lab Notebook/2007-08-23|the day before]], two samples were prepared: |
| - | + | ||
* 2ml of suspension was taken to prepare an interface according to protocol. | * 2ml of suspension was taken to prepare an interface according to protocol. | ||
* 250μl of 100x diluted GFP solution was used to prepare the emulsion; stirred gently with magnetic stirrer. | * 250μl of 100x diluted GFP solution was used to prepare the emulsion; stirred gently with magnetic stirrer. | ||
Latest revision as of 21:05, 26 October 2007

24 August 2007
In Vitro Testing of pLux 25oC
Tested for the working condition of the DNA construct pTet-LuxR-pLux-GFP. This experiment will carry on until fluorescence reaches that of the control.
Protocol can be found here under [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Experimental_Design/Phase1/Protocol_2.2 Phase 1-In vitro testing] on the experimental design page.
Results can here under [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Experimental_Design/Phase1/Results_2.2 Results] on the experimental design page
Preparation of Reaction Buffer for S30 Cell Extract
- Phosphoenolpyruvate has been added
- 30 µl of buffer aliquotes put into eppendorf tubes
- Home-made cell extract (S30) ready to be tested
- Reaction mixture:
- S30 cell extract 16.2 µl
- Reaction buffer 30 µl
- Puruvate kinase 3.1 µl
- rNTPs 1 µl
- DNA 4 µl
- ddH2O 5.7 µl
- Total volume: 60 µl
In Vitro Testing of pTet 25oC using Home-Made Cell Extract with Commercial Pre-Incubation Mix
Tested for the viability of the home-made cell extract.
Protocol followed was according to the Promega one, and can be found here under [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Experimental_Design/Phase1/Protocol_2.1 Phase 1-In vitro testing] on the experimental design page.
Results: It was observed that no fluorescence was produced during the incubation period of over 4 hours.
In Vitro Testing of pTet 25oC using Home-Made Cell Extract and Reaction Buffer
Tested for the viability of the home-made cell extract and reaction buffer.
Protocol followed was according to the one in the paper.
Results: It was observed that no fluorescence was produced during the incubation period of over 4 hours.
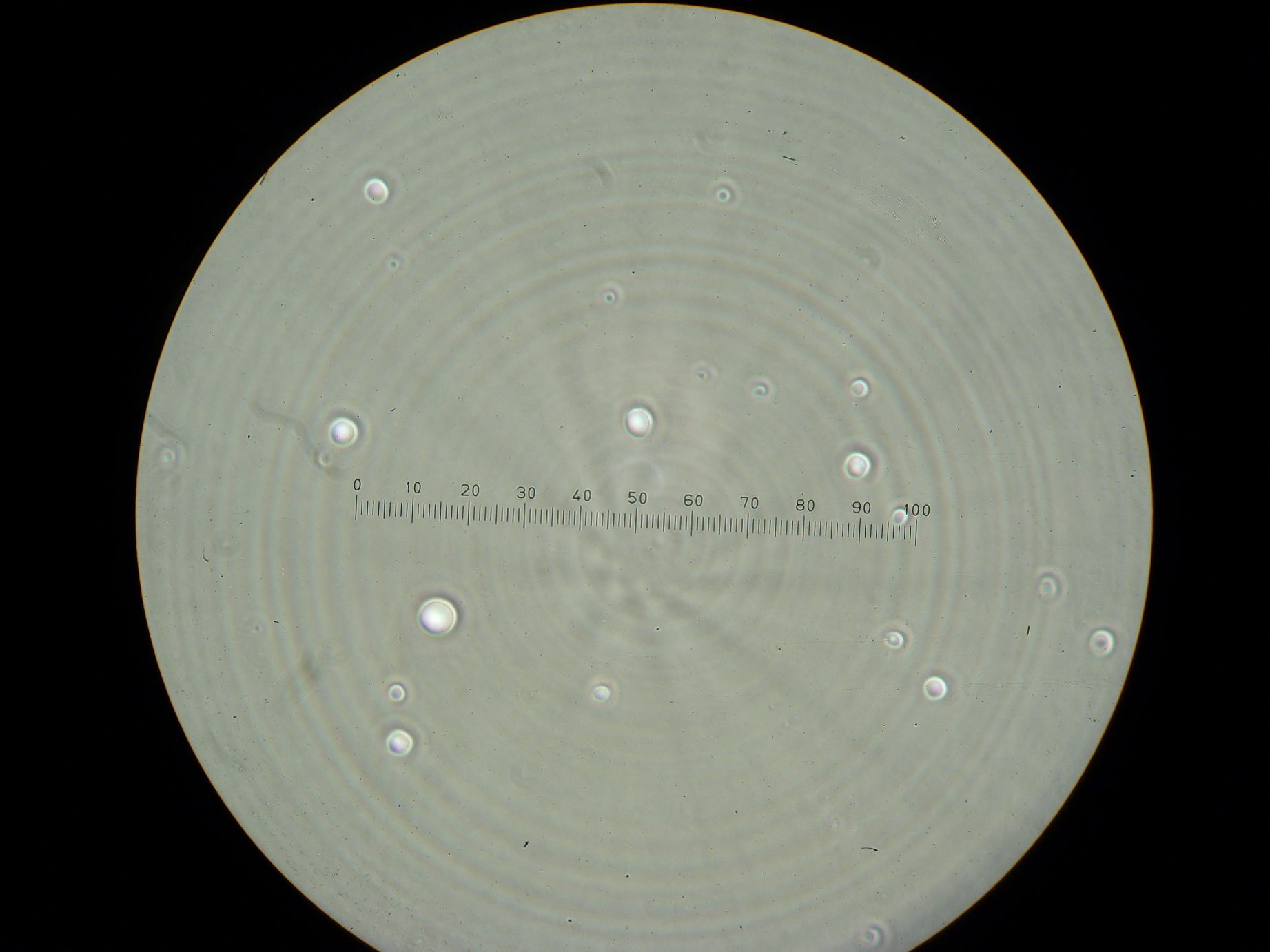
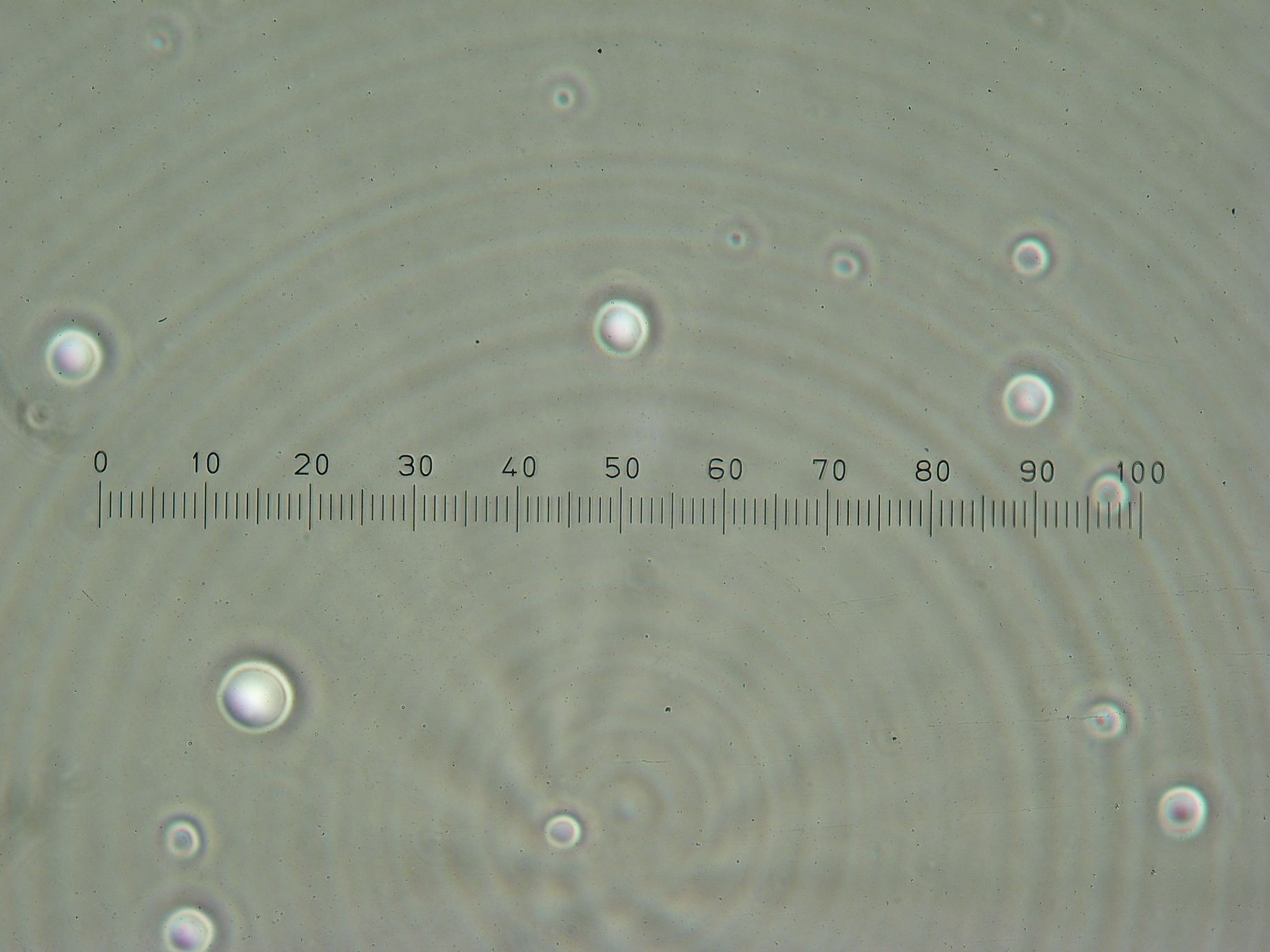
Vesicles Formation with GFP
Formation of Vesicles Using POPC/dodecane suspension from the day before, two samples were prepared:
- 2ml of suspension was taken to prepare an interface according to protocol.
- 250μl of 100x diluted GFP solution was used to prepare the emulsion; stirred gently with magnetic stirrer.
- Special care was taken to protect the GFP solution from light at all times.
In addition, another suspension was prepared using Span-80 and mineral oil. The Span-80 was simply added to the mineral oil and mixed for 10 minutes with a magnetic stir bar before 250μl of 100x diluted GFP solution was added to it.
3 samples prepared:
- Sample 1: 2ml of POPC/dodecane/GFP emulsion, with no previously prepared interface.
- Sample 2: 1ml of POPC/dodecane/GFP emulsion added over interface prepared with 2ml of POPC/dodecane suspension.
- Sample 3: 2ml of Span-80/mineral oil/GFP emulsion, with no previously prepared interface.
All samples were centrifuged at 120x g.
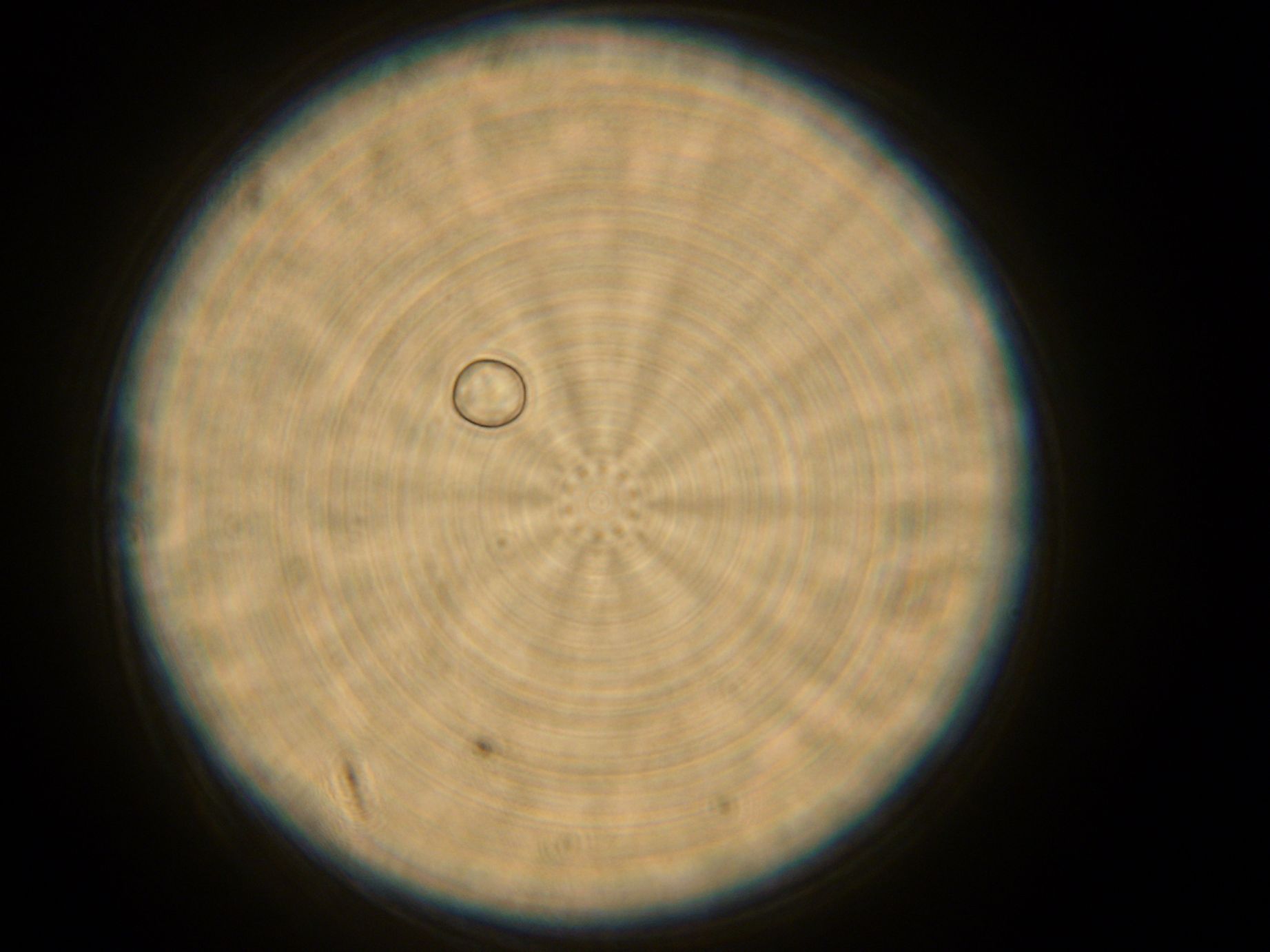
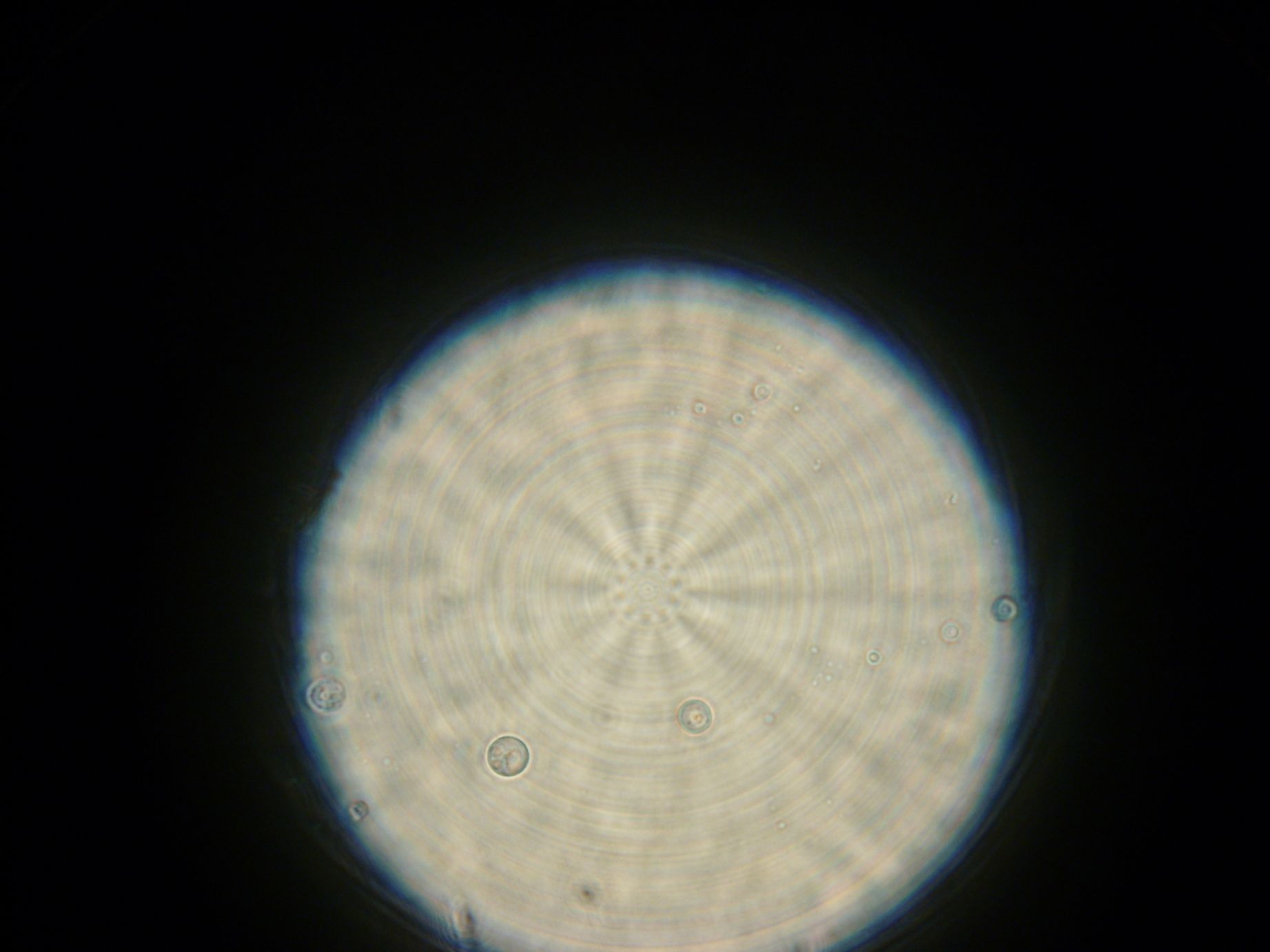
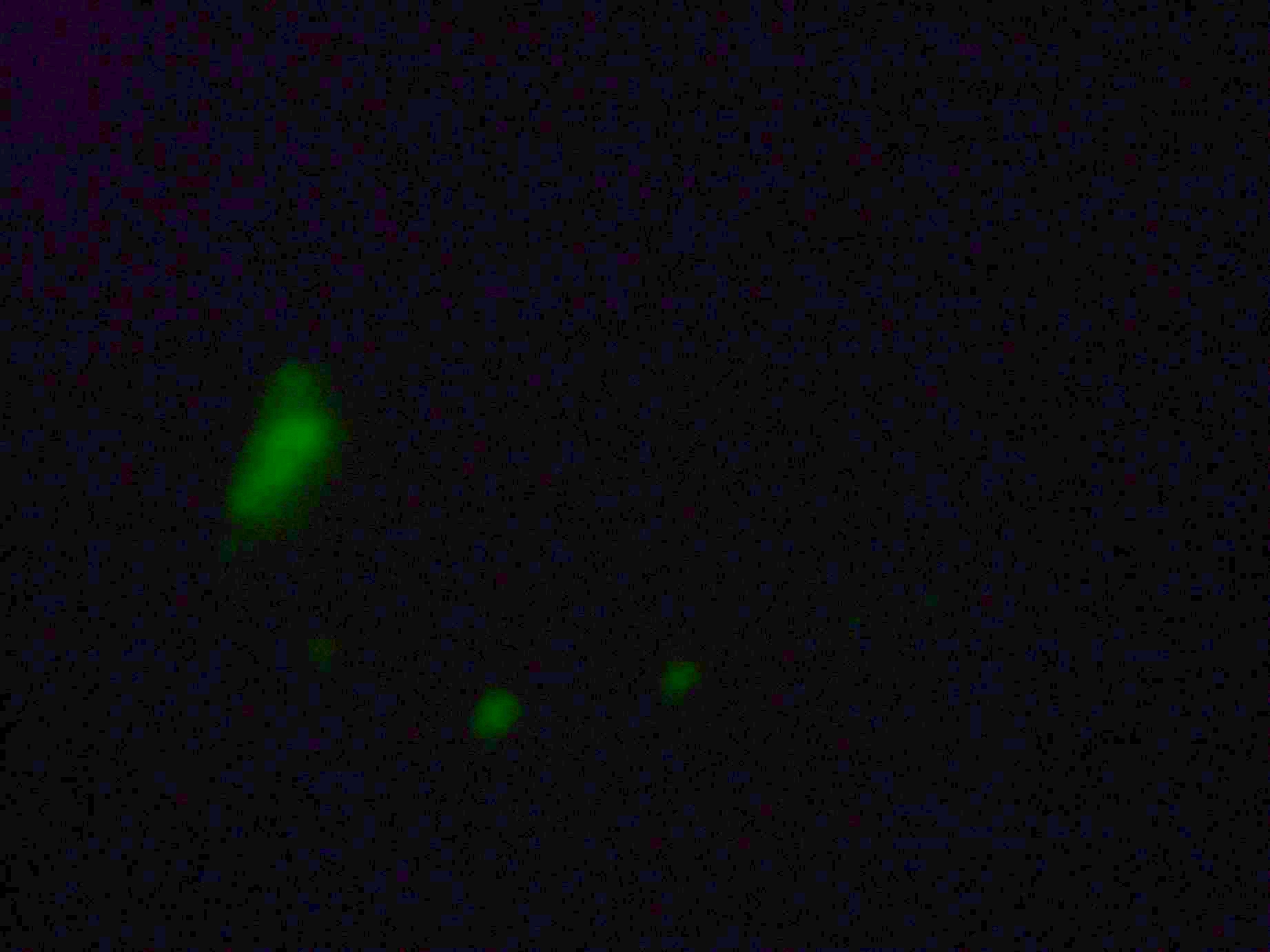
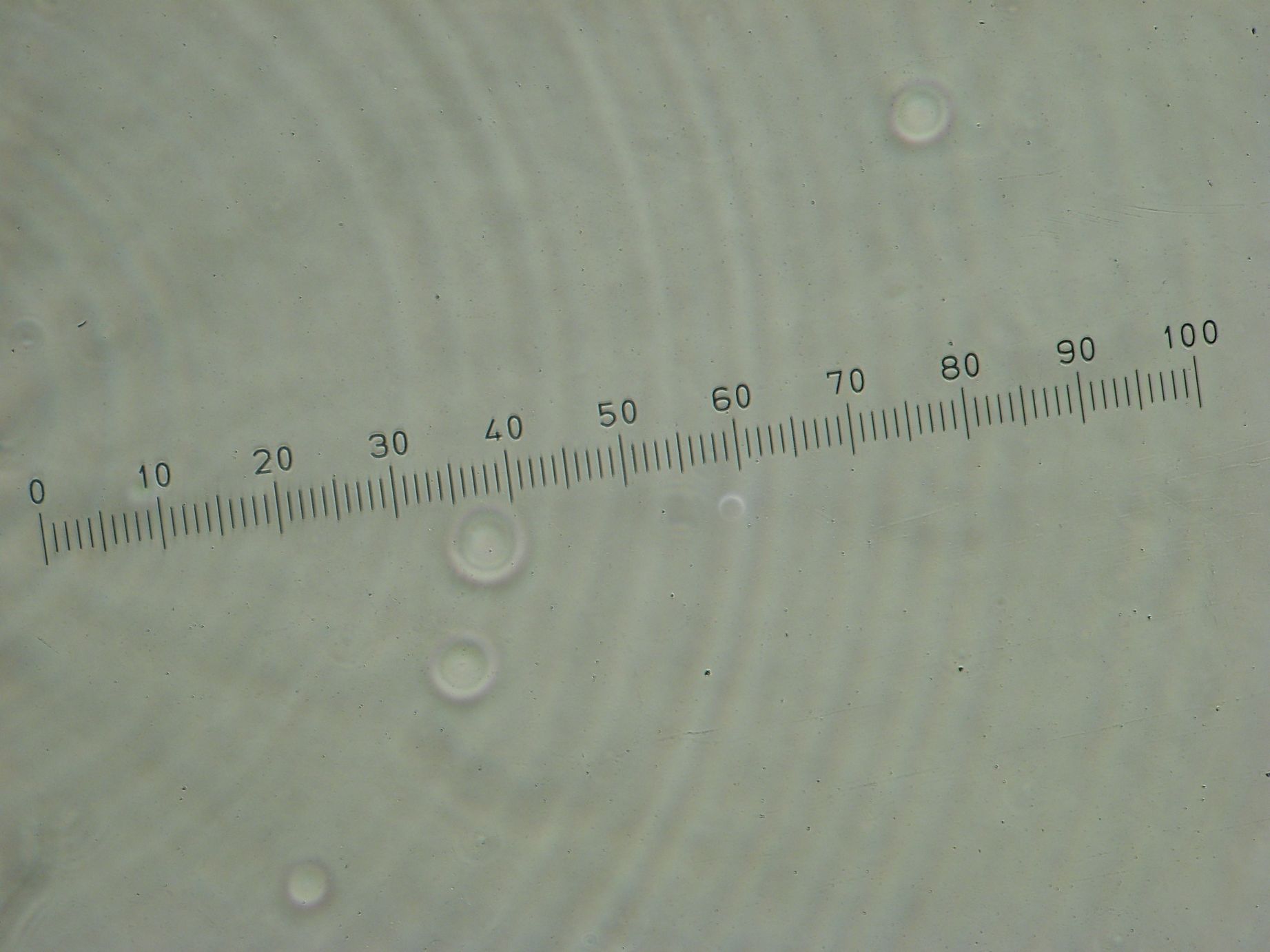
Samples from both the POPC/dodecane and Span-80/mineral oil emulsions were also collected for observation under the microscope.
Results
- Sample 1: Vesicles observed, but very sparse, with no fluorescence.
- Sample 2: Numerous small vesicles encapsulating GFP were observed.
- Sample 3: Vesicles observed, but with no fluorescence. Vesicles were very mobile.
- POPC/dodecane Emulsion: Fluorescent esicles observed, but very sparse.
- Span-80/mineral oil Emulsion: Numerous small vesicles encapsulating GFP were observed.
Preparations
No preparations were carried out.