Imperial/Wet Lab/Lab Notebook/2007-08-22
From 2007.igem.org
(→22 August 2007) |
|||
| Line 5: | Line 5: | ||
| + | ==Midiprep of Biobricks== | ||
| + | Midipreped 6 Biobricks | ||
| + | |||
| + | <font size=-2> | ||
| + | *2. BBa_I13522 [ptet-GFP] | ||
| + | *21. BBa_J23039 [ptet-luxI] | ||
| + | *23. BBa_I13504 [GFP] | ||
| + | *24. BBa_R0062 [pLux (no luxR)] | ||
| + | *25. BBa_J37032 [pLux-GFP] | ||
| + | *26. BBa_S03119 [tet-LuxR]</font> | ||
| + | |||
| + | Protocols can be found at [[IGEM:IMPERIAL/2007/Notebook/General_Protocols#Midiprep|Midiprep]] in the general protocols page | ||
| + | |||
| + | ==Miniprep of Biobrick== | ||
| + | Minipreped 2x1 Biobrick <font size=-2> | ||
| + | *14. BBa_R0051 [pcI]</font> | ||
| + | |||
| + | Protocols can be found at [[IGEM:IMPERIAL/2007/Notebook/General_Protocols#Miniprep|Miniprep]] in the general protocols page | ||
| + | |||
| + | ==Restriction Digest of Biobricks== | ||
| + | #4 DNA samples were digested by EcoRI and PstI | ||
| + | #* Samples 2, 14a, 14b, 24 | ||
| + | #4 DNA samples were digested by Xbai | ||
| + | #* Samples 21, 23, 25, 26 | ||
| + | #4 μl of DNA was digested by 1 μl of enzymes at 37°C for 1 hour | ||
| + | |||
| + | ==Agarose Gel Electrophoresis of Biobricks== | ||
| + | #Checked 7 Biobricks and 7 Digests on 1% agarose gel | ||
| + | |||
| + | Protocols can be found at Protocols can be found at [[IGEM:IMPERIAL/2007/Notebook/General_Protocols#Electrophoresis|Electrophoresis]] in the general protocols page | ||
| + | |||
| + | [[Image:ICGEMS Gel 22 8.jpg]] | ||
| + | |||
| + | Conclusions: | ||
| + | *Part 14 is confirmed to be faulty | ||
| + | *Part 21 does not have the right size | ||
| + | |||
| + | ==In Vivo Testing of pT7 (Continued)== | ||
| + | Continue testing pT7 in vivo at 37<sup>o</sup>C. This test was started on 21-08-2007 and has been left over night. | ||
| + | <br><br> | ||
| + | Protocol can be found here under [[IGEM:IMPERIAL/2007/Experimental_Design/Phase1/Protocol_1.1|Phase 1-In vivo testing]] on the experimental design page. | ||
| + | <br><br> | ||
| + | Results can here under [[IGEM:IMPERIAL/2007/Experimental Design/Phase1/Results 2.1 | Results]] on the experimental design page | ||
| + | |||
| + | ==In Vitro Testing of pTet 37<sup>o</sup>C (Continued)== | ||
| + | Continued with the testing of pTet in vitro which was started on 21-08-2007 at 11.30pm. This experiment will carry on until fluorescence reaches that of the control. | ||
| + | <br><br> | ||
| + | Protocol can be found here under [[IGEM:IMPERIAL/2007/Experimental_Design/Phase1/Protocol_2.1|Phase 1-In vitro testing]] on the experimental design page. | ||
| + | <br><br> | ||
| + | Results can here under [[IGEM:IMPERIAL/2007/Experimental_Design/Phase1/Results_2.1| Results]] on the experimental design page | ||
| + | |||
| + | ==In vitro Testing of pT7 37<sup>o</sup>C== | ||
| + | Initial testing of pT7 in vitro at 37<sup>o</sup>C. This experiment will carry on until fluorescence reaches that of the control. | ||
| + | <br><br> | ||
| + | Protocol can be found here under [[IGEM:IMPERIAL/2007/Experimental_Design/Phase1/Protocol_2.1|Phase 1-In vitro testing]] on the experimental design page. | ||
| + | <br><br> | ||
| + | Results can here under [[IGEM:IMPERIAL/2007/Experimental_Design/Phase1/Results_2.1| Results]] on the experimental design page | ||
| + | |||
| + | ==Operating Range of in vitro Testing of pTet== | ||
| + | Initial testing of operating ranges of pTet in vitro at 10<sup>o</sup>C and 45<sup>o</sup>C. The sampling of this was every 30minutes. | ||
| + | <br><br> | ||
| + | Protocol can be found here under [[IGEM:IMPERIAL/2007/Experimental_Design/Phase2/Protocol_2.1.1|Phase 2-Operating Temperature Ranges]] on the experimental design page. | ||
| + | <br><br> | ||
| + | Results can here under ... on the experimental design page | ||
| + | |||
| + | |||
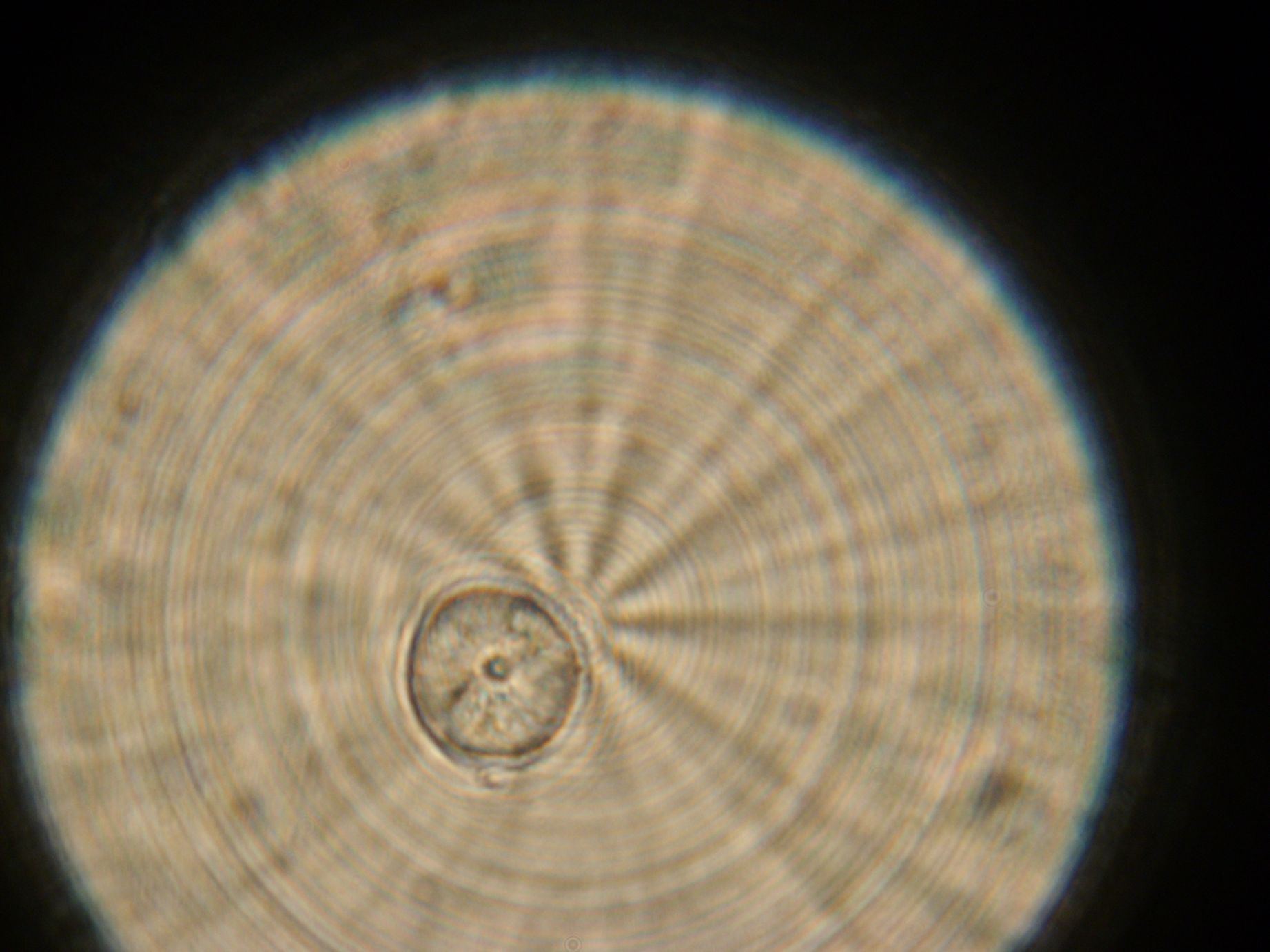
| + | ==Vesicles Formation with GFP== | ||
| + | |||
| + | {|align="right" border="1" | ||
| + | | width="200px"| [[image:IC07_image095.jpg|200px]] | ||
| + | | width="200px"| [[image:IC07_image096.jpg|200px]] | ||
| + | |- | ||
| + | |colspan="2" width="400px"|Microscope pictures from sample 4 (2ml emulsion, centrifuge for 20min at 30x g). Left: A fluorescent object. Right: The same object under white light. | ||
| + | |- | ||
| + | | width="200px"| [[image:IC07_image086.jpg|200px]] | ||
| + | | width="200px"| [[image:IC07_image089.jpg|200px]] | ||
| + | |- | ||
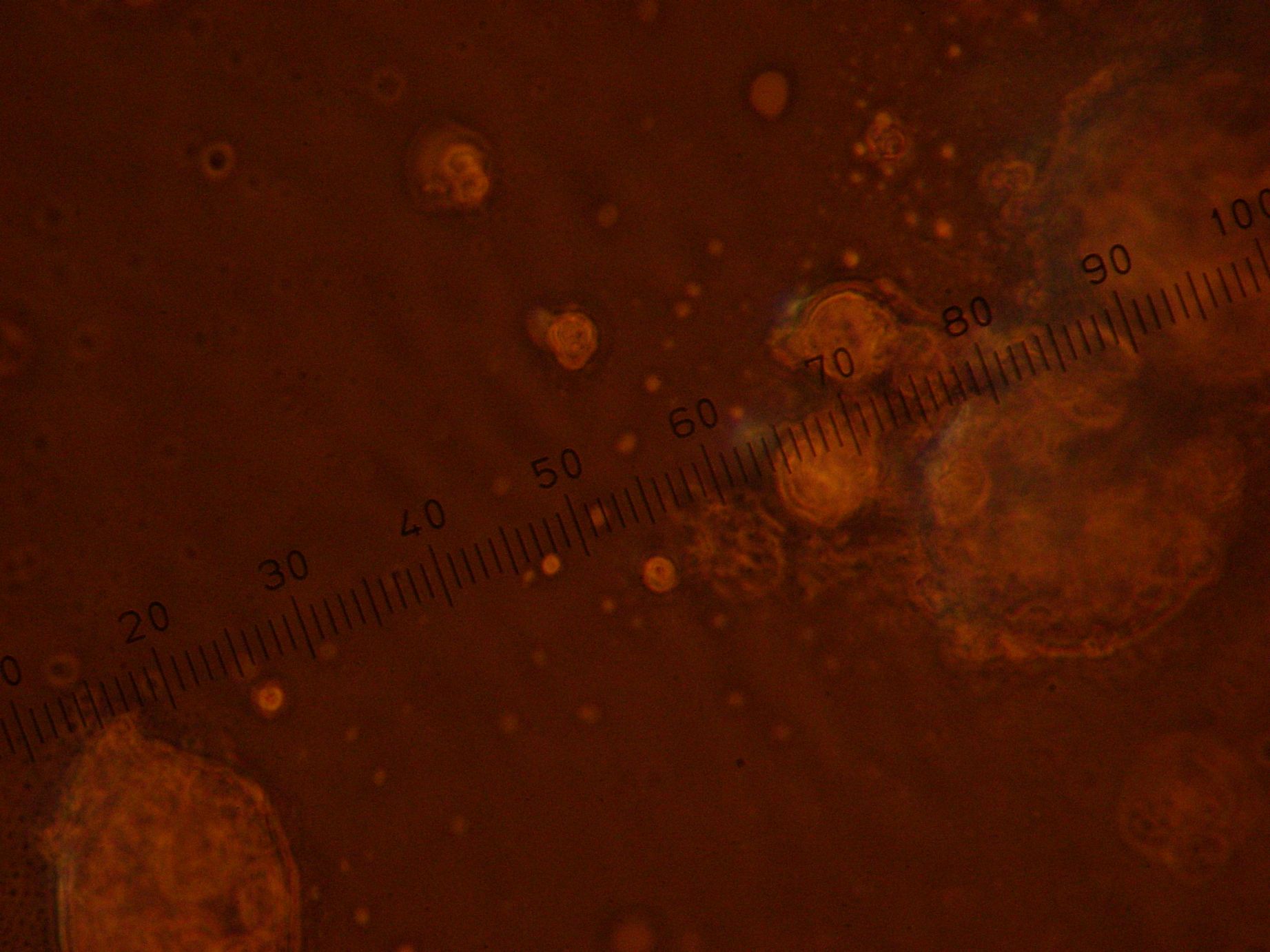
| + | |colspan="2" width="400px"|Strange structures. Left: Aggregates in the oil-lipid emulsion. Right: Air-water interface and an unexpected 'bubbly' structure in the aqueous region. | ||
| + | |} | ||
| + | '''Formation of Vesicles''' | ||
| + | |||
| + | Using a prepared well-dessicated, well sonicated DOPC/mineral oil suspension from [[IGEM:IMPERIAL/2007/Notebook/2007-8-21#Pilot Preparation of Vesicles | yesterday]], | ||
| + | * 2ml of suspension was taken to prepare an interface according to protocol. | ||
| + | * 10x diluted GFP solution was used to prepare the emulsion; stirred gently with magnetic stirrer. | ||
| + | * Special care was taken to protect the GFP solution from light at all times. | ||
| + | |||
| + | 4 samples prepared: | ||
| + | * Sample 1: 2ml of emulsion, no centrifuge. | ||
| + | * Sample 2: Following the protocol, with 2ml suspension interface and 100μl of emulsion added. | ||
| + | * Sample 3: 2ml of emulsion, centrifuge at 120x g for 10mins. | ||
| + | * Sample 4: 2ml of emulsion, centrifuge at 30x g for 20mins. | ||
| + | |||
| + | |||
| + | '''Results''' | ||
| + | |||
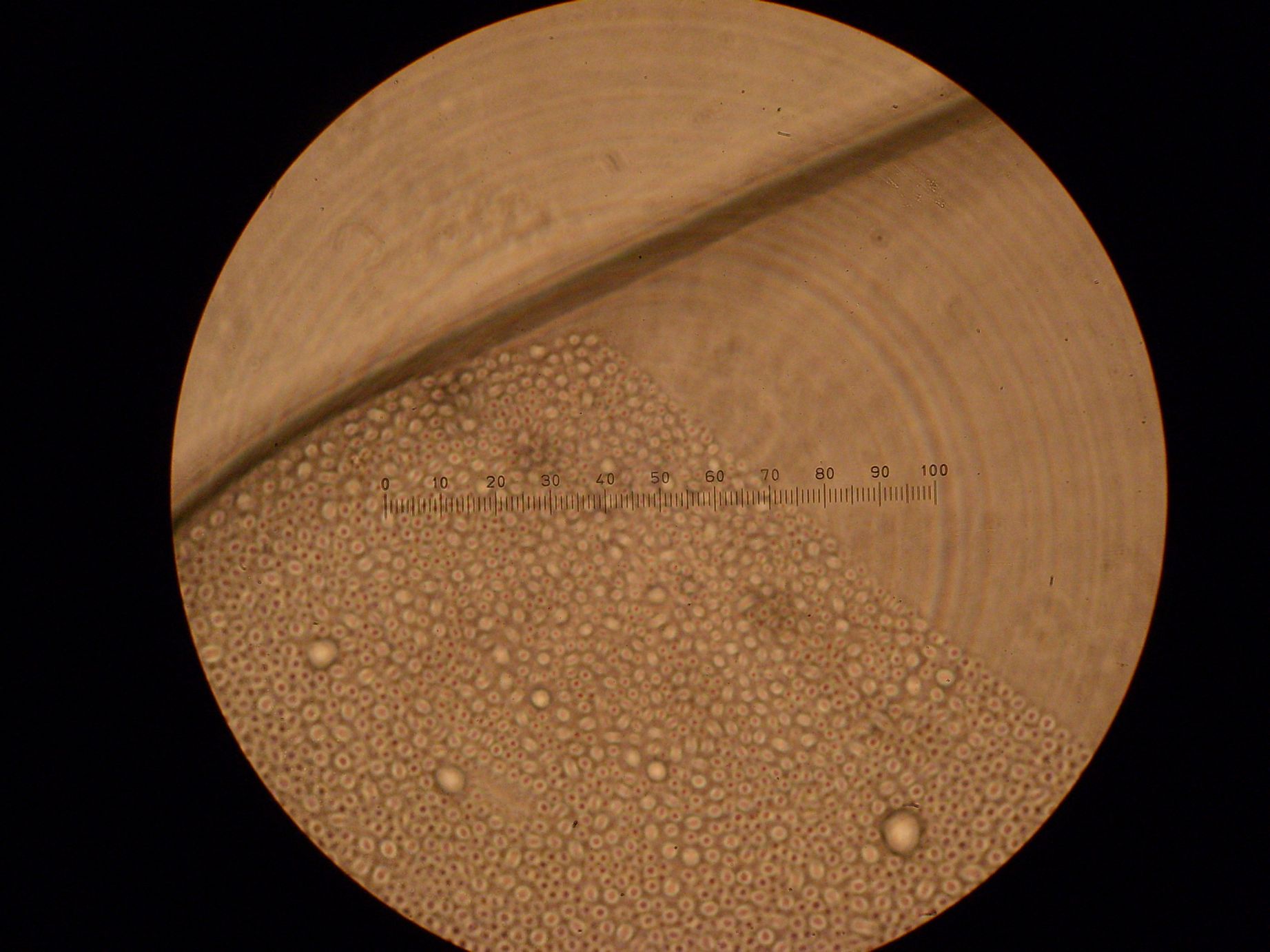
| + | Light Microscopy (Magnification - 100x): | ||
| + | * Emulsion: Vesicles seen, presumably monolayer. | ||
| + | * Sample 1: Water-oil interface observed. Presumably the phospholipids have clumped altogether. | ||
| + | * Sample 2: Vesicles seen. | ||
| + | * Sample 3: Vesicles seen. | ||
| + | * Sample 4: Vesicles seen. | ||
| + | |||
| + | Fluorescence Microscopy (Magnification - 100x): | ||
| + | * Emulsion: GFP vesicles seen. Lots of GFP artifacts. | ||
| + | * Sample 1: No fluorescent vesicles observed. | ||
| + | * Sample 2: No fluorescent vesicles observed. | ||
| + | * Sample 3: "Vesicles" of sizes 1-10 μm found encapsulating GFP within. Lots of GFP artifacts. | ||
| + | * Sample 4: "Vesicles" of sizes 1-10 μm found encapsulating GFP within. Lots of GFP artifacts. | ||
| + | |||
| + | |||
| + | '''Preparations''' | ||
| + | |||
| + | No preparations were carried out. | ||
<html></div></html> | <html></div></html> | ||
{{Template:IC07labnotebook}} | {{Template:IC07labnotebook}} | ||
Revision as of 14:02, 10 October 2007

22 August 2007
Midiprep of Biobricks
Midipreped 6 Biobricks
- 2. BBa_I13522 [ptet-GFP]
- 21. BBa_J23039 [ptet-luxI]
- 23. BBa_I13504 [GFP]
- 24. BBa_R0062 [pLux (no luxR)]
- 25. BBa_J37032 [pLux-GFP]
- 26. BBa_S03119 [tet-LuxR]
Protocols can be found at Midiprep in the general protocols page
Miniprep of Biobrick
Minipreped 2x1 Biobrick
- 14. BBa_R0051 [pcI]
Protocols can be found at Miniprep in the general protocols page
Restriction Digest of Biobricks
- 4 DNA samples were digested by EcoRI and PstI
- Samples 2, 14a, 14b, 24
- 4 DNA samples were digested by Xbai
- Samples 21, 23, 25, 26
- 4 μl of DNA was digested by 1 μl of enzymes at 37°C for 1 hour
Agarose Gel Electrophoresis of Biobricks
- Checked 7 Biobricks and 7 Digests on 1% agarose gel
Protocols can be found at Protocols can be found at Electrophoresis in the general protocols page
Conclusions:
- Part 14 is confirmed to be faulty
- Part 21 does not have the right size
In Vivo Testing of pT7 (Continued)
Continue testing pT7 in vivo at 37oC. This test was started on 21-08-2007 and has been left over night.
Protocol can be found here under Phase 1-In vivo testing on the experimental design page.
Results can here under Results on the experimental design page
In Vitro Testing of pTet 37oC (Continued)
Continued with the testing of pTet in vitro which was started on 21-08-2007 at 11.30pm. This experiment will carry on until fluorescence reaches that of the control.
Protocol can be found here under Phase 1-In vitro testing on the experimental design page.
Results can here under Results on the experimental design page
In vitro Testing of pT7 37oC
Initial testing of pT7 in vitro at 37oC. This experiment will carry on until fluorescence reaches that of the control.
Protocol can be found here under Phase 1-In vitro testing on the experimental design page.
Results can here under Results on the experimental design page
Operating Range of in vitro Testing of pTet
Initial testing of operating ranges of pTet in vitro at 10oC and 45oC. The sampling of this was every 30minutes.
Protocol can be found here under Phase 2-Operating Temperature Ranges on the experimental design page.
Results can here under ... on the experimental design page
Vesicles Formation with GFP
Formation of Vesicles
Using a prepared well-dessicated, well sonicated DOPC/mineral oil suspension from yesterday,
- 2ml of suspension was taken to prepare an interface according to protocol.
- 10x diluted GFP solution was used to prepare the emulsion; stirred gently with magnetic stirrer.
- Special care was taken to protect the GFP solution from light at all times.
4 samples prepared:
- Sample 1: 2ml of emulsion, no centrifuge.
- Sample 2: Following the protocol, with 2ml suspension interface and 100μl of emulsion added.
- Sample 3: 2ml of emulsion, centrifuge at 120x g for 10mins.
- Sample 4: 2ml of emulsion, centrifuge at 30x g for 20mins.
Results
Light Microscopy (Magnification - 100x):
- Emulsion: Vesicles seen, presumably monolayer.
- Sample 1: Water-oil interface observed. Presumably the phospholipids have clumped altogether.
- Sample 2: Vesicles seen.
- Sample 3: Vesicles seen.
- Sample 4: Vesicles seen.
Fluorescence Microscopy (Magnification - 100x):
- Emulsion: GFP vesicles seen. Lots of GFP artifacts.
- Sample 1: No fluorescent vesicles observed.
- Sample 2: No fluorescent vesicles observed.
- Sample 3: "Vesicles" of sizes 1-10 μm found encapsulating GFP within. Lots of GFP artifacts.
- Sample 4: "Vesicles" of sizes 1-10 μm found encapsulating GFP within. Lots of GFP artifacts.
Preparations
No preparations were carried out.