Imperial/Wet Lab/Lab Notebook/2007-08-24
From 2007.igem.org
m |
m (→Vesicles Formation with GFP) |
||
| Line 46: | Line 46: | ||
==Vesicles Formation with GFP== | ==Vesicles Formation with GFP== | ||
'''Formation of Vesicles''' | '''Formation of Vesicles''' | ||
| - | Using POPC/dodecane suspension from [[Imperial/Wet Lab/Lab Notebook/2007-08- | + | Using POPC/dodecane suspension from [[Imperial/Wet Lab/Lab Notebook/2007-08-23|the day before]], two samples were prepared: |
| - | + | ||
* 2ml of suspension was taken to prepare an interface according to protocol. | * 2ml of suspension was taken to prepare an interface according to protocol. | ||
* 250μl of 100x diluted GFP solution was used to prepare the emulsion; stirred gently with magnetic stirrer. | * 250μl of 100x diluted GFP solution was used to prepare the emulsion; stirred gently with magnetic stirrer. | ||
Revision as of 12:54, 26 October 2007

24 August 2007
In Vitro Testing of pLux 25oC
Tested for the working condition of the DNA construct pTet-LuxR-pLux-GFP. This experiment will carry on until fluorescence reaches that of the control.
Protocol can be found here under Phase 1-In vitro testing on the experimental design page.
Results can here under Results on the experimental design page
Preparation of Reaction Buffer for S30 Cell Extract
- Phosphoenolpyruvate has been added
- 30 µl of buffer aliquotes put into eppendorf tubes
- Home-made cell extract (S30) ready to be tested
- Reaction mixture:
- S30 cell extract 16.2 µl
- Reaction buffer 30 µl
- Puruvate kinase 3.1 µl
- rNTPs 1 µl
- DNA 4 µl
- ddH2O 5.7 µl
- Total volume: 60 µl
In Vitro Testing of pTet 25oC using Home-Made Cell Extract with Commercial Pre-Incubation Mix
Tested for the viability of the home-made cell extract.
Protocol followed was according to the Promega one, and can be found here under Phase 1-In vitro testing on the experimental design page.
Results: It was observed that no fluorescence was produced during the incubation period of over 4 hours.
In Vitro Testing of pTet 25oC using Home-Made Cell Extract and Reaction Buffer
Tested for the viability of the home-made cell extract and reaction buffer.
Protocol followed was according to the one in the paper.
Results: It was observed that no fluorescence was produced during the incubation period of over 4 hours.
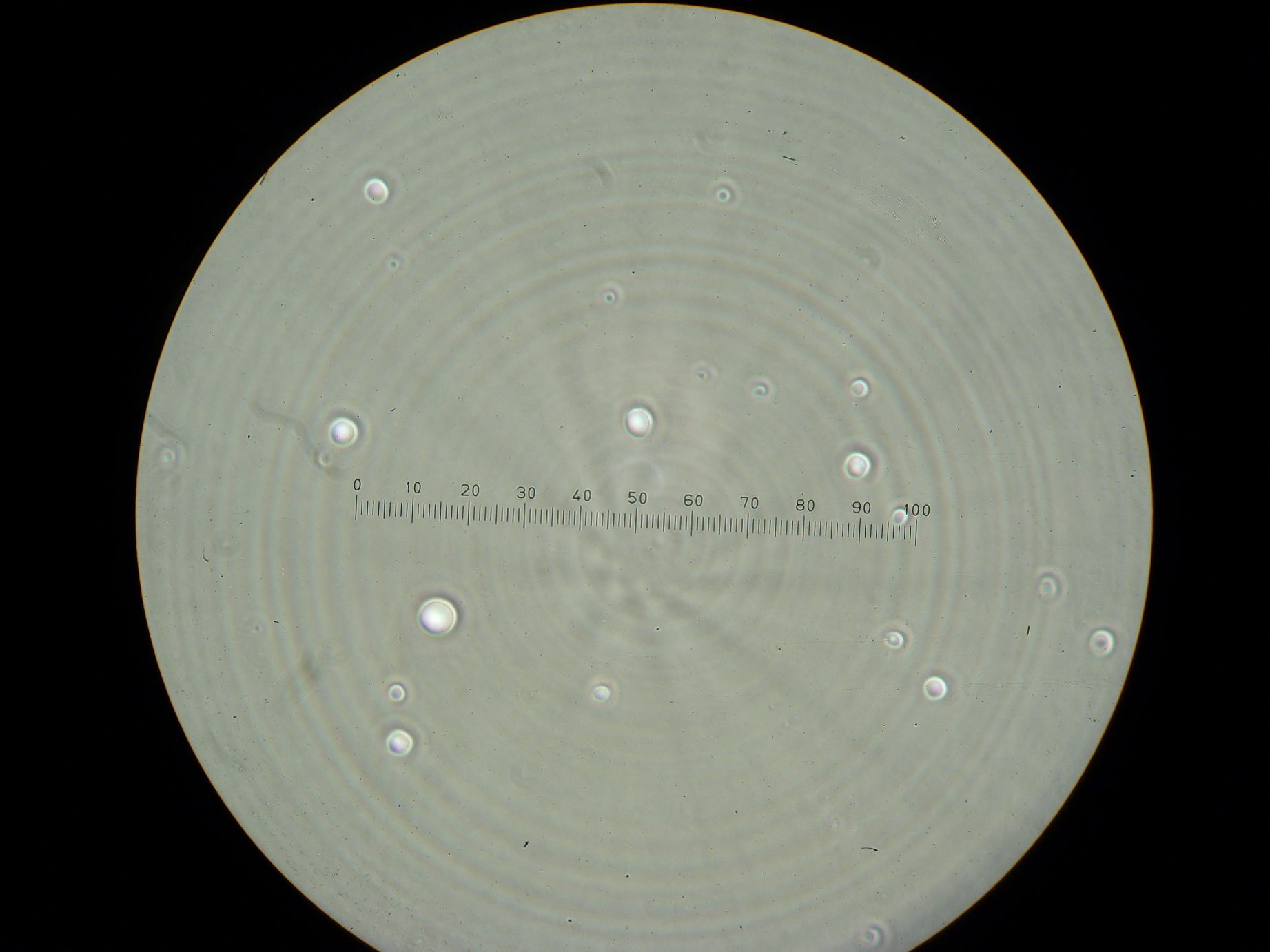
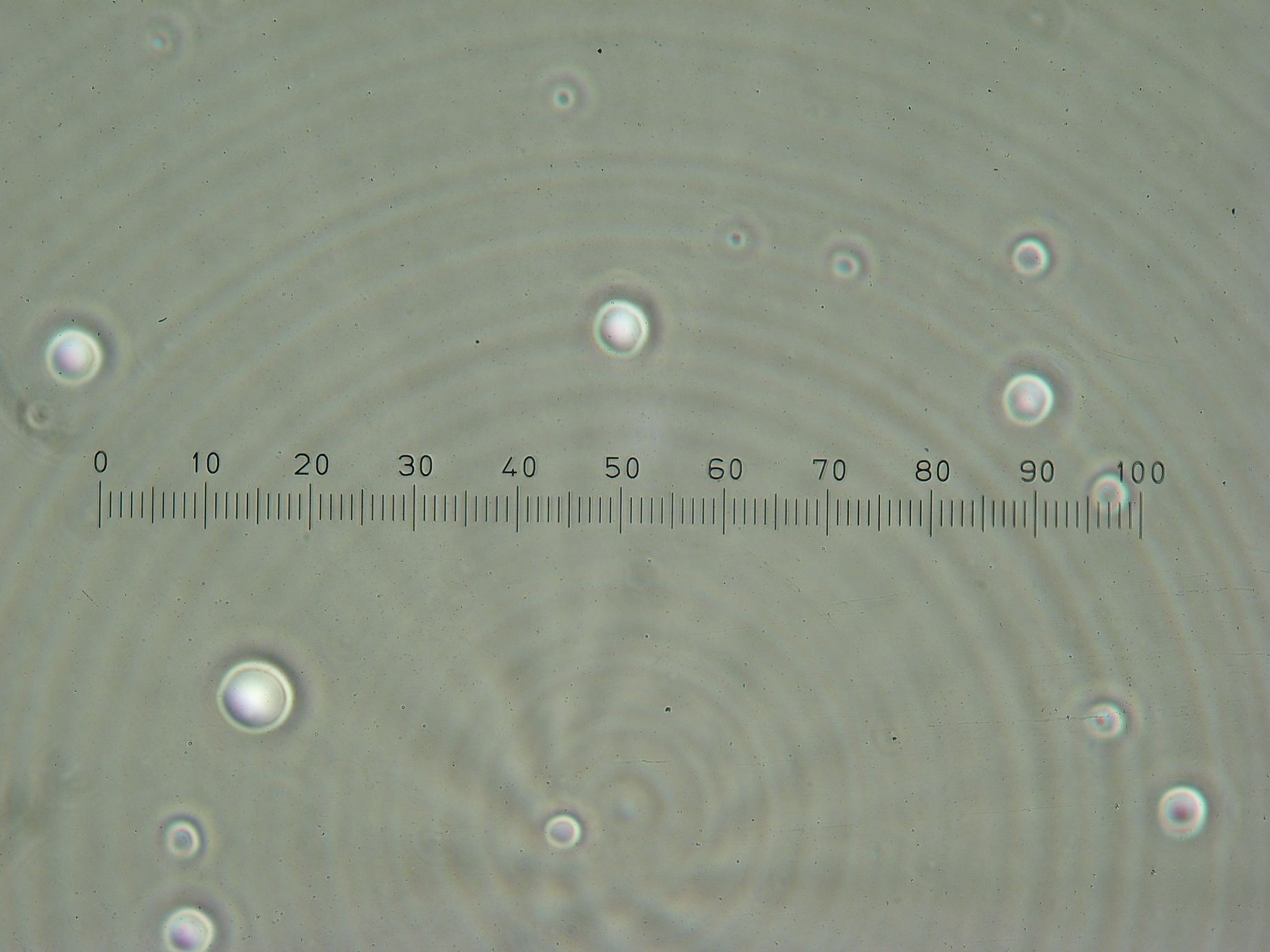
Vesicles Formation with GFP
Formation of Vesicles Using POPC/dodecane suspension from the day before, two samples were prepared:
- 2ml of suspension was taken to prepare an interface according to protocol.
- 250μl of 100x diluted GFP solution was used to prepare the emulsion; stirred gently with magnetic stirrer.
- Special care was taken to protect the GFP solution from light at all times.
In addition, another suspension was prepared using Span-80 and mineral oil. The Span-80 was simply added to the mineral oil and mixed for 10 minutes with a magnetic stir bar before 250μl of 100x diluted GFP solution was added to it.
3 samples prepared:
- Sample 1: 2ml of POPC/dodecane/GFP emulsion, with no previously prepared interface.
- Sample 2: 1ml of POPC/dodecane/GFP emulsion added over interface prepared with 2ml of POPC/dodecane suspension.
- Sample 3: 2ml of Span-80/mineral oil/GFP emulsion, with no previously prepared interface.
All samples were centrifuged at 120x g.
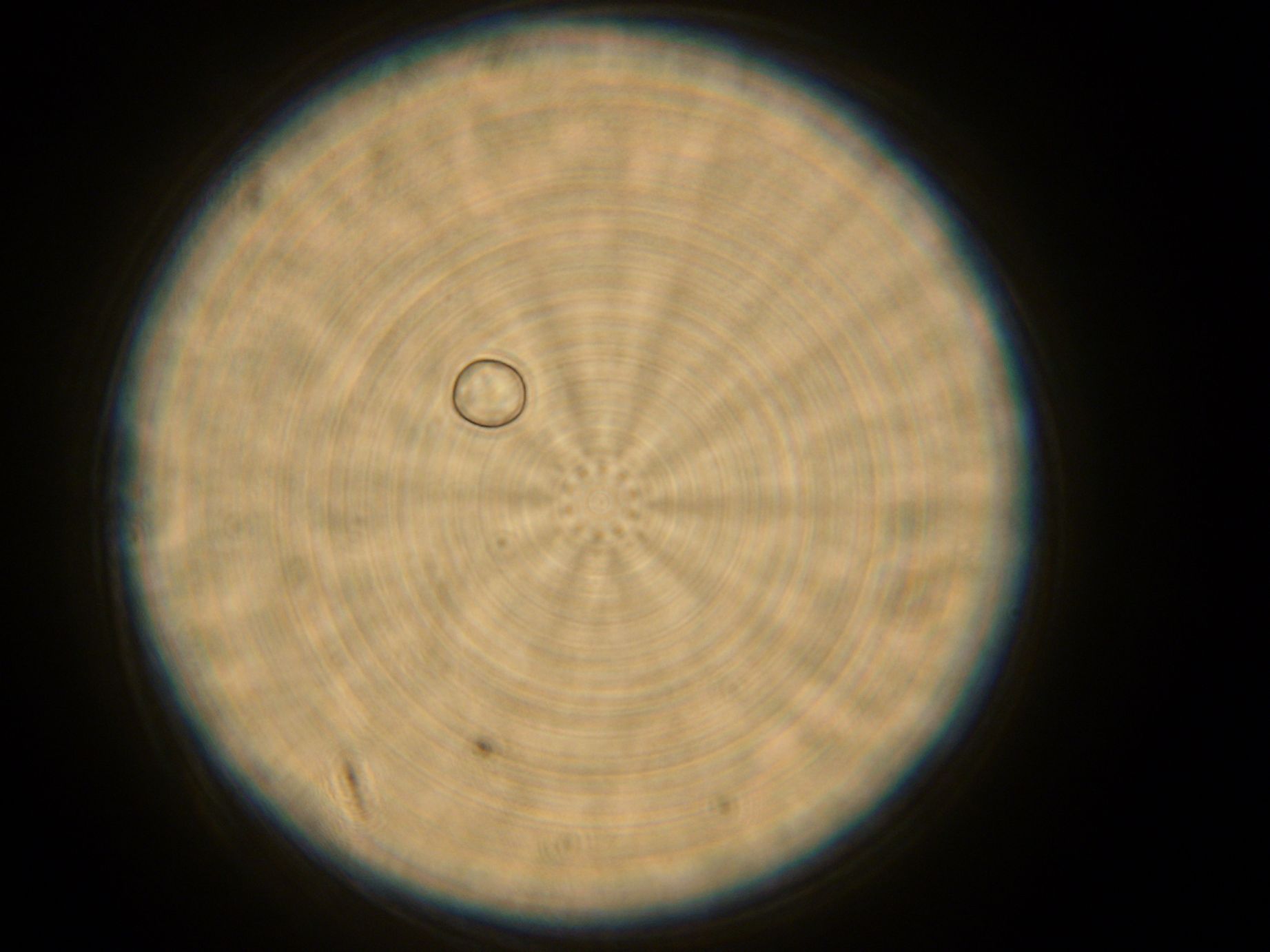
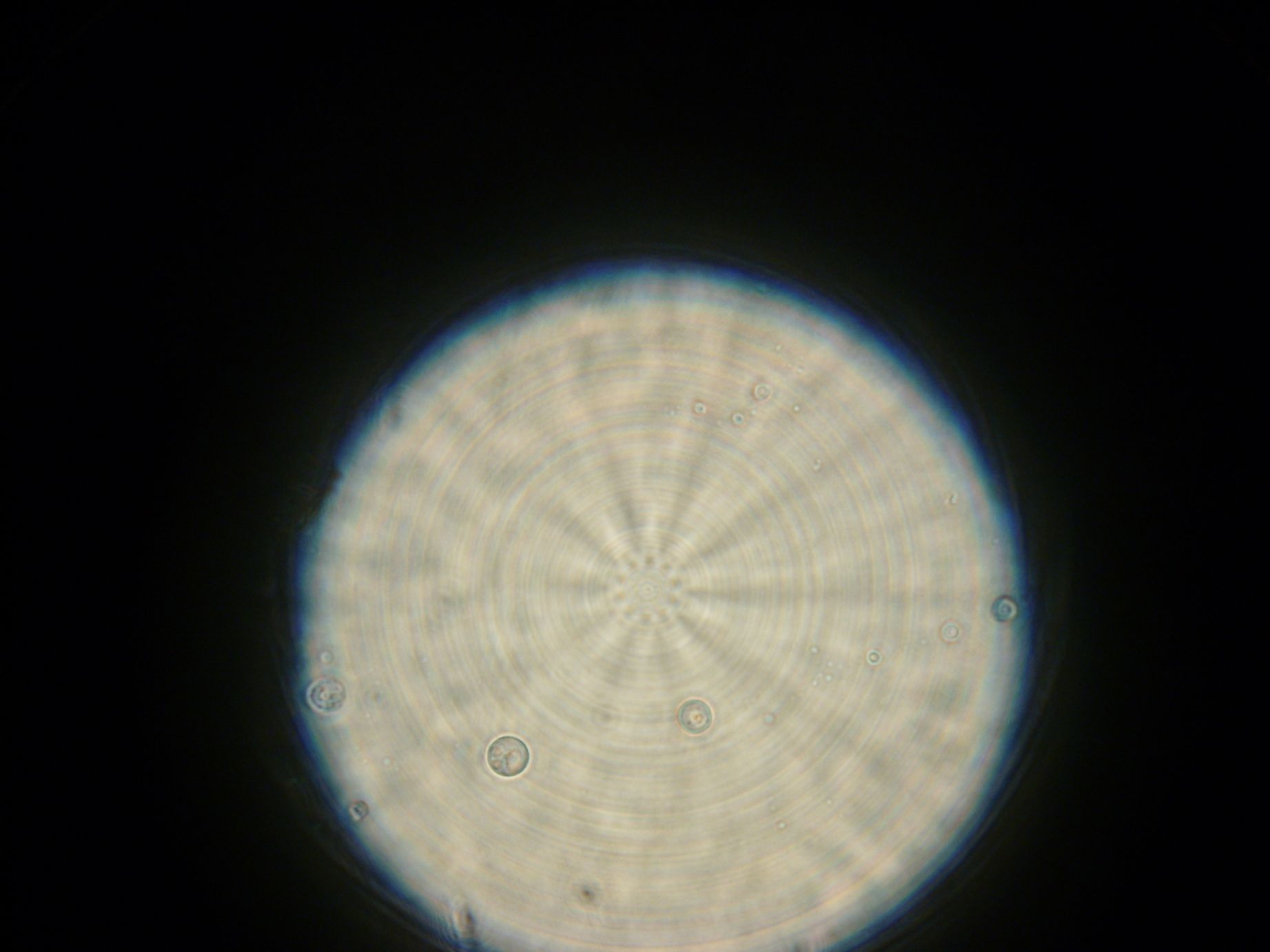
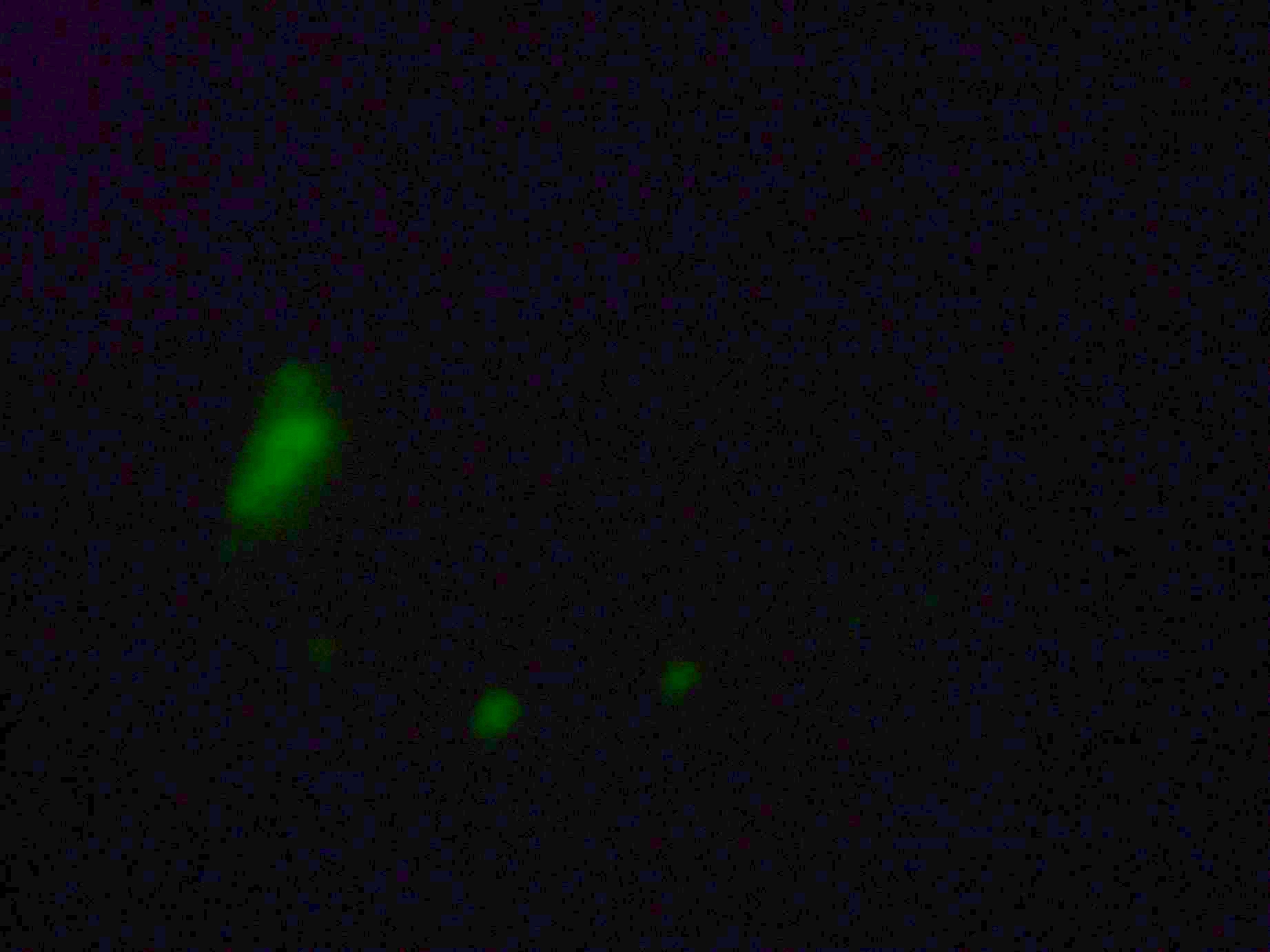
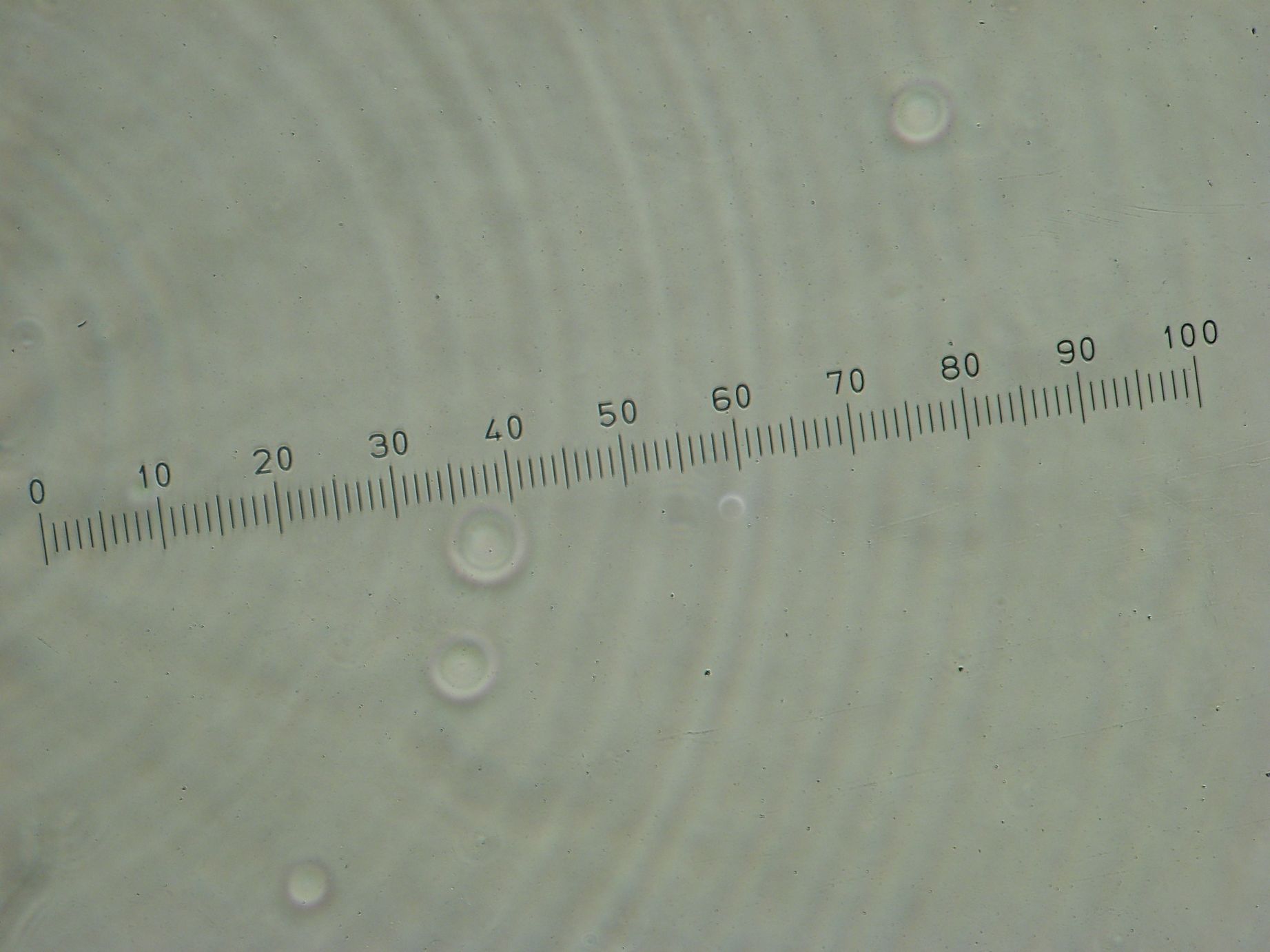
Samples from both the POPC/dodecane and Span-80/mineral oil emulsions were also collected for observation under the microscope.
Results
- Sample 1: Vesicles observed, but very sparse, with no fluorescence.
- Sample 2: Numerous small vesicles encapsulating GFP were observed.
- Sample 3: Vesicles observed, but with no fluorescence. Vesicles were very mobile.
- POPC/dodecane Emulsion: Fluorescent esicles observed, but very sparse.
- Span-80/mineral oil Emulsion: Numerous small vesicles encapsulating GFP were observed.
Preparations
No preparations were carried out.