Imperial/Wet Lab/Lab Notebook/2007-08-17
From 2007.igem.org
(Difference between revisions)
m (→Agarose Gel Electrophoresis of Biobricks) |
m (→Agarose Gel Electrophoresis of Biobricks) |
||
| Line 12: | Line 12: | ||
#Checked 9 Biobricks and 9 Digests (lebelled R) on 1% agarose gel | #Checked 9 Biobricks and 9 Digests (lebelled R) on 1% agarose gel | ||
| - | Protocols can be found at [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Notebook/General_Protocols#S30.2FS12_Cell_Extract#Electrophoresis | + | Protocols can be found at [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Notebook/General_Protocols#S30.2FS12_Cell_Extract#Electrophoresis Electrophoresis] in the general protocols page |
[[Image:IC07 Gel 17 8.jpg]]<br clear="all"> | [[Image:IC07 Gel 17 8.jpg]]<br clear="all"> | ||
Revision as of 19:56, 26 October 2007

17 August 2007
Restriction Digest of Biobricks
- 9 DNA samples were digested by EcoRI and PstI
- 4 μl of DNA was digested by 1 μl of enzymes at 37°C for 1 hour
Agarose Gel Electrophoresis of Biobricks
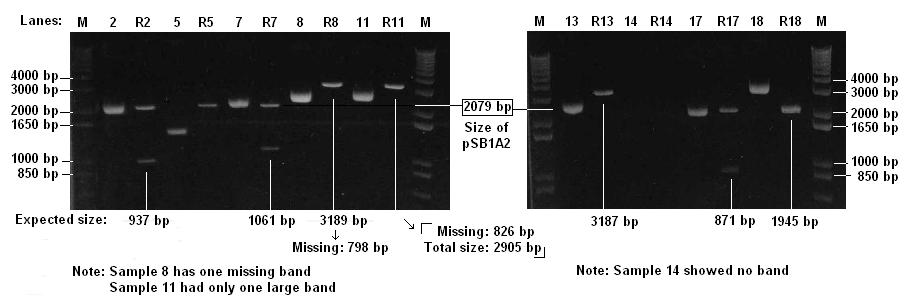
- Checked 9 Biobricks and 9 Digests (lebelled R) on 1% agarose gel
Protocols can be found at [http://www.openwetware.org/wiki/IGEM:IMPERIAL/2007/Notebook/General_Protocols#S30.2FS12_Cell_Extract#Electrophoresis Electrophoresis] in the general protocols page
- The brick for cI promoter (sample 14) is likely to be faulty
- There might be a problem with one of the restriction sites of T7-GFP (sample 11)
Retesting of pT7 in vivo
Today we restested the pT7 in vivo, ensureing that we properly induced it. The results were that there was no expression of GFP from pT7. From these results we will carry out further investigation
Testing of DNA Constructs in vitro
- The following constructs were tested:
- pT7 in Vivo (200 microLitres in each well) -3 repeats
- pTet in Vitro (Homemade cell extract: 100 microL of cell extract + 10 microL of DNA) - 2 repeats
- pT7 in Vitro (Homemade cell extract: 100 microL of cell extract + 10 microL of DNA) - 2 repeats
- Only 2 repeats were carried out to conserve homemade cell extract
- The experiment was carried out at room temp of 25 oC average
- Measurements were taken every 15 min for 4 h
- The fluorometer counting time of the detector was 0.15 s
Pilot Preparation of Vesicles
- Formation of Vesicles: Two of the four suspensions prepared the day before (one well-desiccated and one poorly-desiccated) were used to form vesicles:
- 2ml of each suspension was used to prepare an interface, according to the protocol
- 200x diluted GFP solution was used to prepare the emulsion
- Additionally, the emulsion prepared the day before (1-day old emulsion) was stirred once again to see if it would yield vesicles.
- Another sample from the vesicles prepared the day before was taken for observation under the fluorescence microscope
- 200x diluted GFP solution was also observed under the fluorescence microscope
- It was found that the GFP solution was too weak to be observed in the microscope, and therefore the emulsions being prepared would not work.
- It was also found that the GFP solution needs to be protected from light in order to avoid rapid degradation.
- Upon these discoveries, 10x diluted GFP was added to the three emulsions above while they were already being mixed with the 200x diluted GFP.
- Six samples were prepared:
- Three following the protocol, with 2ml suspension interface and 100μl of each of three emulsions added:
- Sample 1: Well desiccated suspension interface with 100μl of emulsion from well desiccated suspension.
- Sample 2: Poorly desiccated suspension interface with 100μl of emulsion from Poorly desiccated suspension.
- Sample 3: Poorly desiccated suspension interface with 100μl of 1-day old emulsion.
- Three using 2ml of emulsion to form the interface, without further addition of material:
- Sample 4: 2ml of emulsion from well desiccated suspension.
- Sample 5: 2ml of emulsion from poorly desiccated suspension.
- Sample 6: 2ml of 1-day old emulsion.
- Results: A fluorescence microscope was used to inspect the samples:
- Sample 1: Tiny and rare specks of fluorescence were seen, only under 100x magnification, but they did not look like vesicles.
- Sample 2: Sample not looked at.
- Sample 3: Sample not looked at.
- Sample 4: One fluorescent vesicle was observed, under 100x magnification, but the GFP was aggregated on the membrane (a green circle, not a green dot).
- Sample 5: Some GFP aggregates were seen under 40x and 100x magnification, but no structures resembling vesicles.
- Sample 6: Sample not looked at.